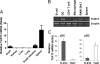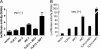A novel Toll-like receptor that recognizes vesicular stomatitis virus - PubMed (original) (raw)
A novel Toll-like receptor that recognizes vesicular stomatitis virus
Zhongcheng Shi et al. J Biol Chem. 2011.
Abstract
Toll-like receptors (TLRs) are the key molecular sensors used by the mammalian innate immune system to detect various types of pathogens. Tlr13 is a novel and uncharacterized member of the mammalian TLR family. Here we report the cloning and characterization of tlr13. Tlr13 is predominantly expressed in the spleen, particularly in dendritic cells and macrophages. Tlr13 appears to activate a MyD88- and TAK1-dependent TLR signaling pathway, inducing the activation of NF-κB. This receptor can also activate type 1 interferon through IRF7. Furthermore, Tlr13 seems to be another intracellular TLR. Remarkably, cells expressing tlr13 fail to respond to known TLR ligands but instead respond specifically to vesicular stomatitis virus. Cells with the knockdown of tlr13 are highly susceptible to vesicular stomatitis virus infection. Thus, these results provide an important insight into the potential role of the novel Toll-like receptor tlr13 in the recognition of viral infection.
Figures
FIGURE 1.
Characterization of TLR13 expression profile. A, real-time RT-PCR analysis of TLR13 mRNA expression in various mouse organs is shown. The graph shows the mean ± S.D. of three independent experiments. B, shown is RT-PCR of TLR13 expression in various cell types in spleen, including B and CD4+ T lymphocytes, DCs, macrophages. Single-cell suspensions of splenocytes were sorted by FACS. β-Actin was used as a control. C, shown is quantitative RT-PCR analysis of TLR13 and TLR7 mRNA expression in pDCs and cDCs.
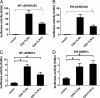
FIGURE 2.
Constitutively active TLR13 (CD4-TLR13) activates both NF-κB and IFN-β. HEK293 cells (A and B) and MEFs (C and D) transiently transfected with expression vectors for CD4-TLR13, CD4-TLR4, or empty expression vector (control) together with an NF-κB or IFN-β luciferase reporter are shown. Luciferase activity was measured with a luminometer after 24 h transfection. Asterisk, p < 0.05. The data are representative of three similar experiments.
FIGURE 3.
Signal transduction by TLR13 is dependent on MyD88 and TAK1. A and B, MyD88−/−, TRIF-siRNA knockdown, TAK1−/−, and wild-type control MEFs were transiently transfected with expression vectors for CD4-TLR13, CD4-TLR3, CD4-TLR4, or empty expression vector (control) together with an IFN-β luciferase reporter. Luciferase activity was measured 48 h after transfection. Asterisk, p < 0.05. The data are representative of three similar experiments. C, MyD88 interacts with TLR13. Immunoblot (IB) analysis of 293 cells expressing TLR13-V5 and MyD88-FLAG directly or after immunoprecipitates (IP) with anti-V5 antibody is shown. D, homodimerization of TLR13 is shown. HEK293 cells were transfected with myc and V5-tagged TLR13 plasmids, TLR13-myc was immunoprecipitated using anti-myc antibody from lysates 48 h post-transfection, and the presence of TLR13-V5 in the immunocomplex was tested.
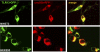
FIGURE 4.
TLR13 localizes intracellularly and interacts with UNC93B. NIH3T3 and HEK293 cells were cotransfected with TLR13-GFP and UNC93B-RFP plasmids. Twenty-four hours after transfection, cells were directly visualized for TLR13-GFP and UNC93B co-localization by confocal microscopy. The images are representative of three independent experiments.
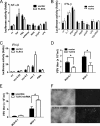
FIGURE 5.
VSV contains TLR13-stimulating activity. A and B, TLR13 does not recognize the known TLR ligands. NIH3T3 cells were transfected with TLR13 or empty expression vector together with NF-κB (A) or IFN-β (B) luciferase reporter. Twenty-four hours after transfection, cells were challenged with different stimuli (10 ng/ml TNF-α, 200 μg/ml phorbol 12-myristate 13-acetate (PMA), 1 μg/ml of single-stranded RNA 40, 1 μg/ml of R848, 10 μg/ml of polyIC, 100 ng/ml LPS, and 10 μg/ml VSV RNA) for 6 h. The luciferase activity was measured with a luminometer. C, the transfected cells were treated with VSV at different multiplicities of infection (0.05, 0.2, and 1) or phorbol 12-myristate 13-acetate (control) for 12 h, and luciferase activity driven by IFN-β promoter was measured. Asterisk, p < 0.05. The data are representative of three similar experiments. D, the transfected NIH3T3 cells were infected with VSV (multiplicity of infection 0.1 and 1), and the virus titer was determined in BHK21 cells 24 h post-infection. E and F, MEFs transiently transfected with pSUPER.retro.puro-scramble or pSUPER.retro.puro-TLR13 shRNA by Lipofectamine 2000 are shown. Twenty-four hours after transfection, cells were infected with VSV for 24 h. Virus titer was determined (E), and the VSV in TLR13 wild-type and knockdown cells were visualized by confocal microscopy (F).
FIGURE 6.
TLR13 activates IFN-β via IRF7, not IRF3 in VSV infection. NIH3T3 cells were transiently transfected with vector, TLR13, IRF3, IRF7, TLR13 plus IRF3, and TLR13 plus IRF7. Luciferase activity was measured after 24 h (A) or challenged with VSV (multiplicity of infection 1) for another 12 h (B).
Similar articles
- Nucleic Acid-Sensing Toll-Like Receptors Play a Dominant Role in Innate Immune Recognition of Pneumococci.
Famà A, Midiri A, Mancuso G, Biondo C, Lentini G, Galbo R, Giardina MM, De Gaetano GV, Romeo L, Teti G, Beninati C. Famà A, et al. mBio. 2020 Mar 24;11(2):e00415-20. doi: 10.1128/mBio.00415-20. mBio. 2020. PMID: 32209688 Free PMC article. - Structurally diverse genes encode TLR13 in Nile tilapia: The two receptors can recognize Streptococcus 23S RNA and conduct signal transduction through MyD88.
Gao FY, Pang JC, Wang M, Lu MX, Liu ZG, Cao JM, Ke XL, Yi MM. Gao FY, et al. Mol Immunol. 2021 Apr;132:60-78. doi: 10.1016/j.molimm.2021.01.020. Epub 2021 Feb 2. Mol Immunol. 2021. PMID: 33545626 - NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2.
Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. Yang Y, et al. J Biol Chem. 2007 Dec 14;282(50):36223-9. doi: 10.1074/jbc.M703079200. Epub 2007 Oct 18. J Biol Chem. 2007. PMID: 17947236 - Role of Toll-like receptor responses for sepsis pathogenesis.
Weighardt H, Holzmann B. Weighardt H, et al. Immunobiology. 2007;212(9-10):715-22. doi: 10.1016/j.imbio.2007.09.010. Epub 2007 Nov 26. Immunobiology. 2007. PMID: 18086373 Review. - TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk.
Brown J, Wang H, Hajishengallis GN, Martin M. Brown J, et al. J Dent Res. 2011 Apr;90(4):417-27. doi: 10.1177/0022034510381264. Epub 2010 Oct 12. J Dent Res. 2011. PMID: 20940366 Free PMC article. Review.
Cited by
- Antiviral immune responses of bats: a review.
Baker ML, Schountz T, Wang LF. Baker ML, et al. Zoonoses Public Health. 2013 Feb;60(1):104-16. doi: 10.1111/j.1863-2378.2012.01528.x. Epub 2012 Aug 1. Zoonoses Public Health. 2013. PMID: 23302292 Free PMC article. Review. - The Pacific Oyster Mortality Syndrome, a Polymicrobial and Multifactorial Disease: State of Knowledge and Future Directions.
Petton B, Destoumieux-Garzón D, Pernet F, Toulza E, de Lorgeril J, Degremont L, Mitta G. Petton B, et al. Front Immunol. 2021 Feb 18;12:630343. doi: 10.3389/fimmu.2021.630343. eCollection 2021. Front Immunol. 2021. PMID: 33679773 Free PMC article. Review. - Endosomal Toll-Like Receptors 7 and 9 Cooperate in Detection of Murine Gammaherpesvirus 68 Infection.
Bussey KA, Murthy S, Reimer E, Chan B, Hatesuer B, Schughart K, Glaunsinger B, Adler H, Brinkmann MM. Bussey KA, et al. J Virol. 2019 Jan 17;93(3):e01173-18. doi: 10.1128/JVI.01173-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429335 Free PMC article. - Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines.
Sartorius R, Trovato M, Manco R, D'Apice L, De Berardinis P. Sartorius R, et al. NPJ Vaccines. 2021 Oct 28;6(1):127. doi: 10.1038/s41541-021-00391-8. NPJ Vaccines. 2021. PMID: 34711839 Free PMC article. Review. - Structural aspects of nucleic acid-sensing Toll-like receptors.
Ohto U, Shimizu T. Ohto U, et al. Biophys Rev. 2016 Mar;8(1):33-43. doi: 10.1007/s12551-015-0187-1. Epub 2016 Feb 12. Biophys Rev. 2016. PMID: 28510149 Free PMC article. Review.
References
- Zhang D., Zhang G., Hayden M. S., Greenblatt M. B., Bussey C., Flavell R. A., Ghosh S. (2004) Science 303, 1522–1526 - PubMed
- Takeda K., Kaisho T., Akira S. (2003) Annu. Rev. Immunol. 21, 335–376 - PubMed
- O'Neill L. A. (2008) Immunity 29, 12–20 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous