Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin - PubMed (original) (raw)
Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin
Haiying Zhang et al. Proc Natl Acad Sci U S A. 2011.
Abstract
In mammals, skin begins as a single-layered epithelium, which, through a series of signals, either stratifies and differentiates to become epidermis or invaginates downward to make hair follicles (HFs). To achieve and maintain proper tissue architecture, keratinocytes must intricately balance growth and differentiation. Here, we uncover a critical and hitherto unappreciated role for Yes-associated protein (YAP), an evolutionarily conserved transcriptional coactivator with potent oncogenic potential. We show that YAP is highly expressed and nuclear in single-layered basal epidermal progenitors. Notably, nuclear YAP progressively declines with age and correlates with proliferative potential of epidermal progenitors. Shortly after initiation of HF morphogenesis, YAP translocates to the cytoplasm of differentiating cells. Through genetic analysis, we demonstrate a role for YAP in maintaining basal epidermal progenitors and regulating HF morphogenesis. YAP overexpression causes hair placodes to evaginate into epidermis rather than invaginate into dermis. YAP also expands basal epidermal progenitors, promotes proliferation, and inhibits terminal differentiation. In vitro gain-and-loss of function studies show that primary mouse keratinocytes (MKs) accelerate proliferation, suppress differentiation, and inhibit apoptosis when YAP is activated and reverse these features when YAP is inhibited. Finally, we identify Cyr61 as a target of YAP in MKs and demonstrate a requirement for TEA domain (TEAD) transcriptional factors to comediate YAP functions in MKs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
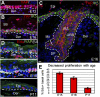
Expression of YAP during embryonic skin development. (A_–_C) Immunofluorescence microscopy of frozen back-skin sections labeled with pan-YAP Ab. (D and E) Progressive decline in nuclear YAP correlates with an age-related reduction in the proliferative potential of basal epidermal progenitors. EdU was administered 4 h before tissue processing and quantifications (n = 8). Primary Abs are color-coded according to their secondary Abs, and nuclei are counterstained with DAPI (blue). (Scale bar, 10 μm.) β4, β4 integrin; B, basal; Der, dermis; DP, dermal papilla; Epi, epidermis; K5, keratin 5 (a pan-marker of basal keratinocytes); SB, suprabasal. Dotted lines denote the dermoepidermal border.
Fig. 2.
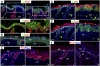
Elevated nuclear YAP results in hyperthickening of interfollicular epidermis and evagination of HFs. (A) Dox-induced Tg pups were distinguished from WT by their lack of a milk spot (*) and open eyes and mouth. (B) H&E staining reveals hyperthickening of Tg interfollicular epidermis accompanied by thinner stratum corneum. (C) HF evaginations in Tg skin are evident by alkaline phosphatase (AP) enzymatic activity (black) in DP. (D) Semithin 1-μm sections of P0 back-skins were stained with toluidine blue, and basal cell density was quantified (as discussed in the text). (E and F) Ultrastructure reveals aberrant cellular morphology in the basal layer and evaginated HFs in Tg mice. B, basal; Der, dermis; DP, dermal papilla; Epi, epidermis; Gr, granular layer; Me, melanin granules (denoted with arrows in F, prevalent in hair shaft and its progenitor Mx cells); Sc, stratum corneum; Sp, spinous layer. (Scale bar, 10 μm.)
Fig. 3.
YAP(S127A) induction leads to an expansion of basal-like layers, increased proliferation, and diminished terminal differentiation. (A–C) Immunofluorescence (IF) reveals enhanced proliferation in P0 YAP Tg epidermis. Note enhanced Ki67- and pH3-positive cells mostly in the basal layer of the epidermis and evaginating HFs but also in some suprabasal cells (arrows in expanded boxed areas). Note also the expanded layers of basal markers K5 and p63 in YAP Tg skin. (D–F) IF shows aberrant terminal differentiation in YAP Tg epidermis. B, basal; Der, dermis; Epi, epidermis; K1, keratin 1; Lor, loricrin; SB, suprabasal. Dotted lines denote the dermoepidermal border. (Scale bar,10 μm.)
Fig. 4.
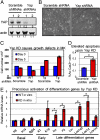
YAP(S127A) induction in MKs in vitro increases proliferation potential and inhibits apoptosis and differentiation. Dox-induced WT and K14-rtTA/TRE-YAP(S127A) Tg MKs were assayed 13 d and 1 d later, respectively, for differences in efficiency to form colonies (A, n = 3) and apoptosis (B, n = 3). Images are phase-contrast. (C) Calcium-induced activation of terminal differentiation genes is suppressed by YAP(S127A) induction in MKs. mRNAs were isolated 24 h after the switch from low to high Ca+, and real-time RT-PCR assays were performed (n = 3). F, feeders. *, statistical significance at the level of P < 0.05.
Fig. 5.
Loss of YAP leads to impaired growth, enhanced cell death, and precocious differentiation of epidermal MKs. Cultured WT and/or K14rtTA/TRE-YAP(S127A) Tg MKs were infected with lentivirus carrying either Yap shRNA or scramble shRNA as a control. Infected MKs were selected with puromycin (2 μg/mL) 2 d after infection and harvested 5 d later. (A) WB analysis reveals efficient KD of YAP expression (β-actin control). (B and C) Differences in cellular morphology (phase-contrast microscopy) and cell growth on Yap KD. Note that the abnormalities can be rescued by activating YAP(S127A). (D) Apoptosis assays. (E) Precocious induction of late-differentiation genes by Yap KD in MKs cultured in low-Ca2+ medium. mRNAs were isolated from FACS-purified MKs 5 d after infection with lentivirus carrying either Yap shRNAs or scramble shRNAs as well as red fluorescent protein (RFP) and were analyzed with real-time RT-PCR (n = 3 in C_–_E). *, statistical significance at the level of P < 0.05.
Fig. 6.
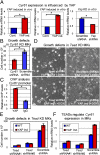
Identification of a target of YAP and of a role for TEADs in mediating YAP function in MKs. (A) Real-time RT-PCR shows that Cyr61 expression is elevated by increased nuclear YAP (ind., induced) in basal epidermal MKs in vivo and in vitro and is repressed by Yap KD (n = 3). (B) Growth defects conferred by Cyr61 KD in MKs. MKs were analyzed 5 d after Cyr61 KD by lentiviral shRNAs (n = 3). (C) ChIP of YAP and IgG (negative control) at the Cyr61 promoter (n = 2). (D and E) Growth defects caused by Tead KD in WT (uninduced, −Dox) and YAP(S127A) overexpressing (induced, +Dox) MKs (n = 3). (F) Effects of Tead KD on Cyr61 expression (n = 3). *, statistical significance at the level of P < 0.05.
Similar articles
- Yap controls stem/progenitor cell proliferation in the mouse postnatal epidermis.
Beverdam A, Claxton C, Zhang X, James G, Harvey KF, Key B. Beverdam A, et al. J Invest Dermatol. 2013 Jun;133(6):1497-505. doi: 10.1038/jid.2012.430. Epub 2012 Nov 29. J Invest Dermatol. 2013. PMID: 23190885 - Plau and Tgfbr3 are YAP-regulated genes that promote keratinocyte proliferation.
Corley SM, Mendoza-Reinoso V, Giles N, Singer ES, Common JE, Wilkins MR, Beverdam A. Corley SM, et al. Cell Death Dis. 2018 Oct 31;9(11):1106. doi: 10.1038/s41419-018-1141-5. Cell Death Dis. 2018. PMID: 30382077 Free PMC article. - Epidermal YAP2-5SA-ΔC Drives β-Catenin Activation to Promote Keratinocyte Proliferation in Mouse Skin In Vivo.
Akladios B, Mendoza-Reinoso V, Samuel MS, Hardeman EC, Khosrotehrani K, Key B, Beverdam A. Akladios B, et al. J Invest Dermatol. 2017 Mar;137(3):716-726. doi: 10.1016/j.jid.2016.10.029. Epub 2016 Nov 2. J Invest Dermatol. 2017. PMID: 27816394 - Advances in resolving the heterogeneity and dynamics of keratinocyte differentiation.
Rice G, Rompolas P. Rice G, et al. Curr Opin Cell Biol. 2020 Dec;67:92-98. doi: 10.1016/j.ceb.2020.09.004. Epub 2020 Oct 19. Curr Opin Cell Biol. 2020. PMID: 33091828 Free PMC article. Review. - Cell differentiation in the embryonic periderm and in scaffolding epithelia of skin appendages.
Eckhart L, Holthaus KB, Sachslehner AP. Eckhart L, et al. Dev Biol. 2024 Nov;515:60-66. doi: 10.1016/j.ydbio.2024.07.002. Epub 2024 Jul 2. Dev Biol. 2024. PMID: 38964706 Review.
Cited by
- Hypoxia and Foxn1 alter the proteomic signature of dermal fibroblasts to redirect scarless wound healing to scar-forming skin wound healing in Foxn1-/- mice.
Gawronska-Kozak B, Machcinska-Zielinska S, Walendzik K, Kopcewicz M, Pääkkönen M, Wisniewska J. Gawronska-Kozak B, et al. BMC Biol. 2024 Sep 11;22(1):193. doi: 10.1186/s12915-024-01990-2. BMC Biol. 2024. PMID: 39256768 Free PMC article. - Can Shockwave Treatment Elicit a Molecular Response to Enhance Clinical Outcomes in Pressure Ulcers? The SHOck Waves in wouNds Project.
Sopel M, Kuberka I, Szczuka I, Taradaj J, Rosińczuk J, Dymarek R. Sopel M, et al. Biomedicines. 2024 Feb 3;12(2):359. doi: 10.3390/biomedicines12020359. Biomedicines. 2024. PMID: 38397961 Free PMC article. - YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer.
Noguchi S, Saito A, Nagase T. Noguchi S, et al. Int J Mol Sci. 2018 Nov 20;19(11):3674. doi: 10.3390/ijms19113674. Int J Mol Sci. 2018. PMID: 30463366 Free PMC article. Review. - Hippo-Yap/Taz signaling: Complex network interactions and impact in epithelial cell behavior.
van Soldt BJ, Cardoso WV. van Soldt BJ, et al. Wiley Interdiscip Rev Dev Biol. 2020 May;9(3):e371. doi: 10.1002/wdev.371. Epub 2019 Dec 11. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31828974 Free PMC article. Review. - The Hippo pathway integrates PI3K-Akt signals with mechanical and polarity cues to control tissue growth.
Borreguero-Muñoz N, Fletcher GC, Aguilar-Aragon M, Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. Borreguero-Muñoz N, et al. PLoS Biol. 2019 Oct 15;17(10):e3000509. doi: 10.1371/journal.pbio.3000509. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31613895 Free PMC article.
References
- Sudol M, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733–14741. - PubMed
- Linn H, et al. Using molecular repertoires to identify high-affinity peptide ligands of the WW domain of human and mouse YAP. Biol Chem. 1997;378:531–537. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous