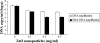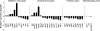Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni - PubMed (original) (raw)
Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni
Yanping Xie et al. Appl Environ Microbiol. 2011 Apr.
Abstract
The antibacterial effect of zinc oxide (ZnO) nanoparticles on Campylobacter jejuni was investigated for inhibition and inactivation of cell growth. The results showed that C. jejuni was extremely sensitive to treatment with ZnO nanoparticles. The MIC of ZnO nanoparticles for C. jejuni was determined to be 0.05 to 0.025 mg/ml, which is 8- to 16-fold lower than that for Salmonella enterica serovar Enteritidis and Escherichia coli O157:H7 (0.4 mg/ml). The action of ZnO nanoparticles against C. jejuni was determined to be bactericidal, not bacteriostatic. Scanning electron microscopy examination revealed that the majority of the cells transformed from spiral shapes into coccoid forms after exposure to 0.5 mg/ml of ZnO nanoparticles for 16 h, which is consistent with the morphological changes of C. jejuni under other stress conditions. These coccoid cells were found by ethidium monoazide-quantitative PCR (EMA-qPCR) to have a certain level of membrane leakage. To address the molecular basis of ZnO nanoparticle action, a large set of genes involved in cell stress response, motility, pathogenesis, and toxin production were selected for a gene expression study. Reverse transcription-quantitative PCR (RT-qPCR) showed that in response to treatment with ZnO nanoparticles, the expression levels of two oxidative stress genes (katA and ahpC) and a general stress response gene (dnaK) were increased 52-, 7-, and 17-fold, respectively. These results suggest that the antibacterial mechanism of ZnO nanoparticles is most likely due to disruption of the cell membrane and oxidative stress in Campylobacter.
Figures
FIG. 1.
Antibacterial activities of ZnO nanoparticles against C. jejuni, S. enterica serovar Enteritidis, and E. coli O157:H7. Freshly grown bacterial cultures (108 to 109 CFU/ml) were treated with a range of concentrations of ZnO nanoparticles. Culturable cell numbers were determined at the time intervals after treatment shown on the figure. The values for CFU/ml are the means of 12 replicates. Error bars indicate standard deviations of the means.
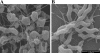
FIG. 2.
Scanning electron microscopic images of C. jejuni. (A) C. jejuni cells in the mid-log phase of growth were treated with 0.5 mg/ml of ZnO nanoparticles for 12 h under microaerobic conditions. (B) Untreated cells from the same growth conditions were used as a control.
FIG. 3.
EMA-qPCR of C. jejuni membrane integrity. Mid-log-phase cells exposed to different concentrations of ZnO nanoparticles were briefly treated with (black bars) and without (white bars) EMA. Inhibition of DNA amplification was quantified by real-time PCR of the hipO gene. Reduced DNA amplification in cells exposed to 0.3 and 0.5 mg/ml of ZnO nanoparticles indicates a certain degree of membrane leakage in the treated cells.
FIG. 4.
Relative gene expression levels of ZnO nanoparticle-treated and untreated C. jejuni. C. jejuni cells in the late log phase of growth were treated with 0 or 0.1 mg/ml of ZnO nanoparticles for 30 min. Transcripts of the selected genes were quantified by RT-qPCR, and data were analyzed using the comparative critical threshold (ΔΔCT) method. The relative expression ratio for each gene is presented as a log2 value in the histogram. A ratio greater than zero (>0) indicates upregulation of gene expression and a ratio below zero (<0) indicates downregulation. Error bars indicate standard deviations for three replicates.
Similar articles
- Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens.
He Y, Ingudam S, Reed S, Gehring A, Strobaugh TP Jr, Irwin P. He Y, et al. J Nanobiotechnology. 2016 Jun 27;14(1):54. doi: 10.1186/s12951-016-0202-0. J Nanobiotechnology. 2016. PMID: 27349516 Free PMC article. - Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella Enteritidis, and Escherichia coli O157:H7.
Jin T, Sun D, Su JY, Zhang H, Sue HJ. Jin T, et al. J Food Sci. 2009 Jan-Feb;74(1):M46-52. doi: 10.1111/j.1750-3841.2008.01013.x. J Food Sci. 2009. PMID: 19200107 - Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7.
Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. Liu Y, et al. J Appl Microbiol. 2009 Oct;107(4):1193-201. doi: 10.1111/j.1365-2672.2009.04303.x. Epub 2009 Apr 17. J Appl Microbiol. 2009. PMID: 19486396 - Examination of nanoparticle inactivation of Campylobacter jejuni biofilms using infrared and Raman spectroscopies.
Lu X, Weakley AT, Aston DE, Rasco BA, Wang S, Konkel ME. Lu X, et al. J Appl Microbiol. 2012 Oct;113(4):952-63. doi: 10.1111/j.1365-2672.2012.05373.x. Epub 2012 Jul 25. J Appl Microbiol. 2012. PMID: 22734855 Free PMC article. - Putative mechanisms and biological role of coccoid form formation in Campylobacter jejuni.
Ikeda N, Karlyshev AV. Ikeda N, et al. Eur J Microbiol Immunol (Bp). 2012 Mar;2(1):41-9. doi: 10.1556/EuJMI.2.2012.1.7. Epub 2012 Mar 17. Eur J Microbiol Immunol (Bp). 2012. PMID: 24611120 Free PMC article. Review.
Cited by
- Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy.
Cleetus CM, Alvarez Primo F, Fregoso G, Lalitha Raveendran N, Noveron JC, Spencer CT, Ramana CV, Joddar B. Cleetus CM, et al. Int J Nanomedicine. 2020 Jul 15;15:5097-5111. doi: 10.2147/IJN.S255937. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32764939 Free PMC article. - Synergistic effects of Cu-doped ZnO nanoantibiotic against Gram-positive bacterial strains.
Khalid A, Ahmad P, Alharthi AI, Muhammad S, Khandaker MU, Faruque MRI, Din IU, Alotaibi MA, Khan A. Khalid A, et al. PLoS One. 2021 May 14;16(5):e0251082. doi: 10.1371/journal.pone.0251082. eCollection 2021. PLoS One. 2021. PMID: 33989295 Free PMC article. - Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach.
Bogdan J, Zarzyńska J, Pławińska-Czarnak J. Bogdan J, et al. Nanoscale Res Lett. 2015 Dec;10(1):1023. doi: 10.1186/s11671-015-1023-z. Epub 2015 Aug 4. Nanoscale Res Lett. 2015. PMID: 26239879 Free PMC article. - Biodegradable and Drug-Eluting Inorganic Composites Based on Mesoporous Zinc Oxide for Urinary Stent Applications.
Laurenti M, Grochowicz M, Dragoni E, Carofiglio M, Limongi T, Cauda V. Laurenti M, et al. Materials (Basel). 2020 Aug 29;13(17):3821. doi: 10.3390/ma13173821. Materials (Basel). 2020. PMID: 32872464 Free PMC article. - Modulation of Biofilm Formation and Permeability in Streptococcus mutans during Exposure To Zinc Acetate.
Buzza KM, Pluen A, Doherty C, Cheesapcharoen T, Singh G, Ledder RG, Sreenivasan PK, McBain AJ. Buzza KM, et al. Microbiol Spectr. 2023 Feb 21;11(2):e0252722. doi: 10.1128/spectrum.02527-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36809043 Free PMC article.
References
- Arsène, F., T. Tomoyasu, and B. Bukau. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55:3-9. - PubMed
- Brayner, R., et al. 2006. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 6:866-870. - PubMed
- Catrenich, C. E., and K. M. Makin. 1991. Characterization of the morphologic conversion of Helicobacter pylori from bacillary to coccoid forms. Scand. J. Gastroenterol. Suppl. 181:58-64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials