Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers - PubMed (original) (raw)
Clinical Trial
. 2011 Jun 10;29(17):2357-63.
doi: 10.1200/JCO.2010.33.9473. Epub 2011 Apr 25.
Mitch A Phelps, Xiaobai Li, Motoyasu Saji, Laura Goff, John Sae Wook Kauh, Bert H O'Neil, Stephanie Balsom, Catherine Balint, Ryan Liersemann, Vasily V Vasko, Mark Bloomston, William Marsh, L Austin Doyle, Gilian Ellison, Michael Grever, Matthew D Ringel, Miguel A Villalona-Calero
Affiliations
- PMID: 21519026
- PMCID: PMC3107751
- DOI: 10.1200/JCO.2010.33.9473
Clinical Trial
Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers
Tanios Bekaii-Saab et al. J Clin Oncol. 2011.
Abstract
Purpose: Biliary cancers (BCs) carry a poor prognosis, but targeting the RAS/RAF/mitogen-activated protein kinase kinase (MEK)/extracellular signal-related kinase (ERK) pathway is of significance. Selumetinib is an inhibitor of MEK1/2, so this trial was designed to determine the safety and efficacy of selumetinib in BC.
Patients and methods: This was a multi-institutional phase II study of selumetinib at 100 mg given orally twice per day to patients with advanced BC. The primary end point was response rate. All patients were required to provide tissue before enrolling. The levels of phosphorylated ERK (pERK) and AKT (pAKT) were assessed by immunohistochemistry. Tumors were genotyped for the presence of BRAF- and/or RAS-activating mutations.
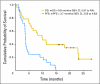
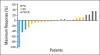
Results: Twenty-eight eligible patients with a median age of 55.6 years were enrolled. Thirty-nine percent of patients had received one prior systemic therapy. Three patients (12%) had a confirmed objective response. Another 17 patients (68%) experienced stable disease (SD), 14 of whom (56%) experienced prolonged SD (> 16 weeks). Patients gained an average nonfluid weight of 8.6 pounds. Median progression-free survival was 3.7 months (95% CI, 3.5 to 4.9) and median overall survival was 9.8 months (95% CI, 5.97 to not available). Toxicities were mild, with rash (90%) and xerostomia (54%) being most frequent. Only one patient experienced grade 4 toxicity (fatigue). All patients had tissue available for analysis. No BRAF V600E mutations were found. Two patients with short-lived SD had KRAS mutations. Absence of pERK staining was associated with lack of response.
Conclusion: Selumetinib displays interesting activity and acceptable tolerability in patients with metastatic BC. Our results warrant further evaluation of selumetinib in patients with metastatic BC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
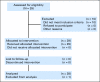
Fig 1.
CONSORT diagram.
Fig 2.
Kaplan-Meier plots for overall survival (OS) and progression-free survival (PFS). mOS, median OS; NA, not available; mPFS, median PFS.
Fig 3.
Waterfall plot showing maximum percentage decrease in target lesions. (*) Patients with 0% change as maximum response. PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
Fig A1.
Representative immunohistochemistry results from patients 13, 7, and 17 to demonstrate the range of staining. pERK, phosphorylated ERK; pAKT, phosphorylated AKT.
Comment in
- An uphill battle downstream of RAF.
Collisson EA. Collisson EA. J Clin Oncol. 2011 Jun 10;29(17):2298-300. doi: 10.1200/JCO.2011.34.9316. Epub 2011 Apr 25. J Clin Oncol. 2011. PMID: 21519020 No abstract available. - Inhibitor of MEK1/2, selumetinib, for biliary tract cancer.
Furuse J, Nagashima F. Furuse J, et al. Expert Rev Gastroenterol Hepatol. 2011 Oct;5(5):579-81. doi: 10.1586/egh.11.58. Expert Rev Gastroenterol Hepatol. 2011. PMID: 21910575
Similar articles
- Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers.
Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG. Adjei AA, et al. J Clin Oncol. 2008 May 1;26(13):2139-46. doi: 10.1200/JCO.2007.14.4956. Epub 2008 Apr 7. J Clin Oncol. 2008. PMID: 18390968 Free PMC article. Clinical Trial. - Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma.
Kirkwood JM, Bastholt L, Robert C, Sosman J, Larkin J, Hersey P, Middleton M, Cantarini M, Zazulina V, Kemsley K, Dummer R. Kirkwood JM, et al. Clin Cancer Res. 2012 Jan 15;18(2):555-67. doi: 10.1158/1078-0432.CCR-11-1491. Epub 2011 Nov 2. Clin Cancer Res. 2012. PMID: 22048237 Free PMC article. Clinical Trial. - Phase II study of selumetinib, an orally active inhibitor of MEK1 and MEK2 kinases, in KRASG12R-mutant pancreatic ductal adenocarcinoma.
Kenney C, Kunst T, Webb S, Christina D Jr, Arrowood C, Steinberg SM, Mettu NB, Kim EJ, Rudloff U. Kenney C, et al. Invest New Drugs. 2021 Jun;39(3):821-828. doi: 10.1007/s10637-020-01044-8. Epub 2021 Jan 6. Invest New Drugs. 2021. PMID: 33405090 Free PMC article. Clinical Trial. - Selumetinib in the treatment of non-small-cell lung cancer.
Bernabé R, Patrao A, Carter L, Blackhall F, Dean E. Bernabé R, et al. Future Oncol. 2016 Nov;12(22):2545-2560. doi: 10.2217/fon-2016-0132. Epub 2016 Jul 28. Future Oncol. 2016. PMID: 27467210 Review. - Selumetinib in advanced non small cell lung cancer (NSCLC) harbouring KRAS mutation: endless clinical challenge to KRAS-mutant NSCLC.
Paolo M, Assunta S, Antonio R, Claudia SP, Anna BM, Clorinda S, Francesca C, Fortunato C, Cesare G. Paolo M, et al. Rev Recent Clin Trials. 2013 Jun;8(2):93-100. doi: 10.2174/15748871113089990047. Rev Recent Clin Trials. 2013. PMID: 24063423 Review.
Cited by
- Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies.
Sia D, Tovar V, Moeini A, Llovet JM. Sia D, et al. Oncogene. 2013 Oct 10;32(41):4861-70. doi: 10.1038/onc.2012.617. Epub 2013 Jan 14. Oncogene. 2013. PMID: 23318457 Free PMC article. Review. - Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies.
Brandi G, Farioli A, Astolfi A, Biasco G, Tavolari S. Brandi G, et al. Oncotarget. 2015 Jun 20;6(17):14744-53. doi: 10.18632/oncotarget.4539. Oncotarget. 2015. PMID: 26142706 Free PMC article. Review. - Chemotherapy for the biliary tract cancers: moving toward improved survival time.
Romiti A, D'Antonio C, Zullo A, Sarcina I, Di Rocco R, Barucca V, Durante V, Marchetti P. Romiti A, et al. J Gastrointest Cancer. 2012 Sep;43(3):396-404. doi: 10.1007/s12029-012-9369-2. J Gastrointest Cancer. 2012. PMID: 22328060 Review. - A phase I, open-label, randomized crossover study to assess the effect of dosing of the MEK 1/2 inhibitor Selumetinib (AZD6244; ARRY-142866) in the presence and absence of food in patients with advanced solid tumors.
Leijen S, Soetekouw PM, Jeffry Evans TR, Nicolson M, Schellens JH, Learoyd M, Grinsted L, Zazulina V, Pwint T, Middleton M. Leijen S, et al. Cancer Chemother Pharmacol. 2011 Dec;68(6):1619-28. doi: 10.1007/s00280-011-1732-7. Epub 2011 Sep 28. Cancer Chemother Pharmacol. 2011. PMID: 21953275 Free PMC article. Clinical Trial. - Gallbladder Cancer in the 21st Century.
Kanthan R, Senger JL, Ahmed S, Kanthan SC. Kanthan R, et al. J Oncol. 2015;2015:967472. doi: 10.1155/2015/967472. Epub 2015 Sep 1. J Oncol. 2015. PMID: 26421012 Free PMC article. Review.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
- Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. - PubMed
- Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. - PubMed
- Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous