Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53 - PubMed (original) (raw)
Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53
Alejandro P Ugalde et al. EMBO J. 2011.
Abstract
Aging is a multifactorial process that affects most of the biological functions of the organism and increases susceptibility to disease and death. Recent studies with animal models of accelerated aging have unveiled some mechanisms that also operate in physiological aging. However, little is known about the role of microRNAs (miRNAs) in this process. To address this question, we have analysed miRNA levels in Zmpste24-deficient mice, a model of Hutchinson-Gilford progeria syndrome. We have found that expression of the miR-29 family of miRNAs is markedly upregulated in Zmpste24(-/-) progeroid mice as well as during normal aging in mouse. Functional analysis revealed that this transcriptional activation of miR-29 is triggered in response to DNA damage and occurs in a p53-dependent manner since p53(-/-) murine fibroblasts do not increase miR-29 expression upon doxorubicin treatment. We have also found that miR-29 represses Ppm1d phosphatase, which in turn enhances p53 activity. Based on these results, we propose the existence of a novel regulatory circuitry involving miR-29, Ppm1d and p53, which is activated in aging and in response to DNA damage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
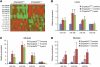
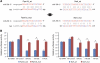
miRNA expression analysis in tissues from _Zmpste24_−/− mice. (A) A total of 17 and 16 _Zmpste24_−/− and Zmpste24+/+ liver samples were analysed through a bead-based flow cytometry platform that covers the complete set of human and mouse miRNAs. miRNAs with significant fold induction were plotted as a colour heat map, with the miR-29 family miRNAs underlined (_P_-value<0.05, 0.75⩾fold induction⩾1.25). (B) miR-29a, -29b and -29c upregulation in _Zmpste24_−/− was confirmed through stem loop qPCR in liver samples. Expression analysis in liver from aged mice shows similar levels of miR-29 miRNAs than in samples from _Zmpste24_−/− mice. As a control, levels of miR-23b that shows no changes in the profiling study were analysed. (C) qPCR analysis of the miR-29 family in muscle revealed eight-fold induction in samples from _Zmpste24_−/− and aged Zmpste24+/+, compared with young _Zmpste24_−/−. (D) Correlation between miR-29 levels and phenotype development in _Zmpste24_−/− muscle samples. For all the experiments, a minimum of three animals were analysed. *Significantly different from 2-month-old wild-type mice, P<0.05.
Figure 2
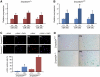
miR-29 expression is associated with DNA damage and cell senescence. Levels of miR-29 increase with serial passages of _Zmpste24_−/− (A) and Zmpste24+/+ (B) primary ear fibroblasts in culture. Total RNA from three independent _Zmpste24_−/− and Zmpste24+/+ fibroblast cell lines was extracted at passages 1 and 6 and the miR-29a, -29b and -29c levels were analysed by qPCR. *Significantly different from passage 1 fibroblasts, P<0.05. (C) Representative images and quantification (lower chart) of γ-H2AX staining in the three _Zmpste24_−/− and Zmpste24+/+ fibroblast cell lines at passages 1 and 6. *Significantly different from passage 1 fibroblasts, P<0.05. (D) Senescence-associated β-galactosidase activity (SA-βGAL) representative images from _Zmpste24_−/− and Zmpste24+/+ primary fibroblasts at passages 1 and 6. Both wild-type and _Zmpste24_-mutant primary fibroblasts show an increase in γ-H2AX foci and SA-βGal activity at passage 6, evidencing the genotoxic stress induced by the in vitro serial passage of primary fibroblasts.
Figure 3
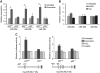
miR-29 induction is linked to DNA damage response. (A) miR-29 expression is activated during DNA damage in a p53-dependent manner. Primary ear fibroblasts from p53+/+ and _p53_−/− mice were treated for 24 h with doxorubicin or left untreated in parallel, and miR-29 expression was analysed by qPCR (_n_=2 biological replicates). (B) Transient damage induced by H2O2 or UV-like lessons caused by 4-nitroquinolone-1-oxide (4-NQO) show no effect on miR-29 transcription. Primary ear fibroblasts were treated with 80 μM H2O2 or 0.8 μM 4-NQO for 24 h and miR-29 levels were analysed by qPCR (_n_=2 biological replicates). Untreated samples in (A) and (B) were taken at the same time points as in treated cells. (C) Transcriptional regulation of miR-29 promoters. Approximately 4 kb of the promoter sequence of both hsa-miR-29b-1∼29a and hsa-miR-29b-2∼29c clusters were cloned upstream the firefly luciferase coding sequence of the pGL3-basic plasmid (diagrams below the chart). Promoter constructs were transfected in wild-type _or p53_−/− HCT-116 cells and treated with 1 μM doxorubicin or left untreated for 36 h. In all transfections, a plasmid expressing Renilla luciferase was included for normalization. The normalized luciferase activity relative to the untreated sample is represented. p53 deficiency abolishes or strongly reduces the promoter activity of hsa-miR-29b-1∼29a and hsa-miR-29b-2∼29c upon doxorubicin treatment, respectively. Additionally, 4-NQO and H2O2 treatments were included as a negative control. *Significantly different from the indicated condition, P<0.05.
Figure 4
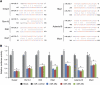
miR-29 target prediction and validation. (A) Pairwise alignment of predicted miR-29 targets. Base pair numbers on each 3′-UTR are indicated. (B) Luciferase assays in HEK-293 cells. The 3′-UTR of each predicted target was cloned downstream of the ORF of Renilla luciferase and transfected into HEK-293 cells alone or together with miR-29a, -29b or -29c or a control miRNA. Data were normalized to the firefly luciferase and experiments were carried out in triplicate. *Significantly different from HEK-293 cells transfected with no miRNA, P<0.05.
Figure 5
Mutation of the miRNA binding site abolishes miRNA repression. (A) Pairwise alignment between miR-29b and the wild-type and mutated 3′-UTR of Narf and Ppm1d (Narf_mut and Ppm1d_mut), in which bases at positions 4 and 5 of the seed region were mutated and are highlighted in grey. (B) Luciferase experiments with the wild-type and the mutated 3′-UTR of Narf and Ppm1d. Mutation of the seed region of the 3′-UTR binding site abolishes the repression triggered by either of the three miR-29 miRNAs, confirming the presence of functional miRNA binding sites in the 3′-UTR of Narf and Ppm1d. *Significantly different, P<0.05.
Figure 6
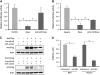
Doxorubicin treatment represses Ppm1d and Narf synthesis through miR-29. Doxorubicin treatment decreases luminescence emission in cells transfected with the wild-type 3′-UTR of Ppm1d (A) and Narf (B). Transfection of miR-29 inhibitory molecules abolishes the translational repression over the 3′-UTRs of Ppm1d and Narf after doxorubicin treatment (_n_=3 biological replicates). (C) Immunoblotting of HEK-293 cells transfected with pcDNA-flag-Narf or pcDNA-flag-Ppm1d in combination with miR-29 microRNA precursor molecules or a microRNA control. The full-length mRNAs of both Narf and Ppm1d, including the 3′-UTR were cloned into pcDNA vector in frame with a flag epitope for immunoblot detection with an anti-Flag antibody. (D) Densitometry analysis of the immunoblots shown in (A). Overexpression of miR-29 reduces the protein levels of Narf and Wip1 by 55 and 72%, respectively. *Significantly different, P<0.05.
Figure 7
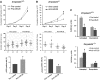
miR-29 overexpression reduces cell proliferation and viability and increases senescence. (A) Effects of miR-29 on cell proliferation and senescence. Passage 2 wild-type primary fibroblasts were transfected with a combination of miR-29a, -29b and -29c or control precursor miRNA (pre-miR-29 or pre-control) A million cells growing in 10 cm dishes were transfected with the indicated molecules and every 3 days cell populations were determined by cell counting using a hemocytometer (top panel). At each passage, a million cells were re-seeded and this procedure was repeated during three passages (three wild-type and three mutant mice fibroblasts were used in each condition). Additionally, SA-βGal activity (middle panel) and γ-H2AX (bottom panel) markers at the end point of the experiment were analysed. (B) The same experiment was carried out with passage 4 _Zmpste24_−/− fibroblast using inhibitory molecules (anti-miR-29 or anti-control). (C) MTT assay of cell viability under doxorubicin treatment. Primary wild-type fibroblasts (top panel) transfected with miR-29 or control precursor molecules and _Zmpste24_-deficient mice fibroblast transfected with miR-29 or control inhibitor molecules (bottom panel) were treated with 1 μM doxorubicin or left untreated for 72 h and cell viability was measured by MTT assay (_n_=3 biological replicates). *Significantly different from pre- or anti-control transfected cells, P<0.05.
Figure 8
miR-29 modulates the DNA damage response through Ppm1d. (A) Wild-type mouse ear fibroblasts were transfected with a combination of pre- or anti-miR-29 molecules, left to recover for 24 h and treated with 0.5 μM doxorubicin or left untreated for 24 h. Then, cells were harvested and phospho-(S15)-p53, total-p53 and β-actin protein levels were analysed by western blot using specific antibodies. (B) Western blot of _Zmpste24_−/− primary fibroblasts transfected with control or miR-29 inhibitor molecules in untreated or doxorubicin-treated conditions. After transfection, cells were left to recover for 24 h, and then treated with 0.5 μM doxorubicin or left untreated for 24 h. (C) Western blot analysis of U2OS cells transfected with a combination of pre-miR-29 or anti-miR-29 molecules. After 48 h, transfection cells were harvested and protein levels were assessed by western blot using anti-PPM1D, anti-phospho-(S15)-p53, anti-total-p53 and anti-β-actin antibodies. (D) Western blot analysis of U2OS cells infected with a lentiviral vector that expresses miR-29-b-c (miR-29) cluster or the empty vector (empty). Cells were treated with 0.1 μM doxorubicin for 90 min and after 3 h cells were harvested and proteins were separated on SDS–PAGE and probed with anti-PPM1D, anti-phospho-(S15)-p53, anti-total-p53 and anti-β-actin antibodies.
Figure 9
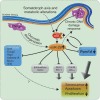
Model for the miR-29 regulatory network in _Zmpste24_−/− progeroid mice. Nuclear lamina alterations of _Zmpste24_−/− cells induce a genomic instability situation that it is recognized by these cells as a chronic DNA damage, thereby activating the p53 signalling pathway. Additionally, nuclear lamina abnormalities disrupt the chromatin–lamina attachment patterns causing profound changes in chromatin structure. These alterations, putatively reinforced by the somatotroph axis and metabolic alterations present in these mice (dotted line), lead to an abnormal miR-29 activation and a subsequent repression of their target genes. These targets include several genes with pro-survival roles, like Bcl-2, Mcl-1, Cdc42, _p85_-α and Ppm1d/Wip1, whose downregulation leads to a decrease of cell proliferation and an increase of cell senescence and apoptosis, processes that finally result in the loss of tissue and organism homoeostasis. Moreover, Ppm1d repression diminishes the ratio of p53 dephosphorylation, which positively feedbacks the p53 loop, exacerbating the initial situation. Additionally, increased miR-29 levels may directly participate in further exacerbating DNA damage. Dotted lines indicate hypothetical links.
Similar articles
- MicroRNA transcriptome analysis identifies miR-365 as a novel negative regulator of cell proliferation in Zmpste24-deficient mouse embryonic fibroblasts.
Xiong XD, Jung HJ, Gombar S, Park JY, Zhang CL, Zheng H, Ruan J, Li JB, Kaeberlein M, Kennedy BK, Zhou Z, Liu X, Suh Y. Xiong XD, et al. Mutat Res. 2015 Jul;777:69-78. doi: 10.1016/j.mrfmmm.2015.04.010. Epub 2015 Apr 24. Mutat Res. 2015. PMID: 25983189 Free PMC article. - Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation.
Varela I, Cadiñanos J, Pendás AM, Gutiérrez-Fernández A, Folgueras AR, Sánchez LM, Zhou Z, Rodríguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, López-Otín C. Varela I, et al. Nature. 2005 Sep 22;437(7058):564-8. doi: 10.1038/nature04019. Epub 2005 Aug 3. Nature. 2005. PMID: 16079796 - The p53/miRNAs/Ccna2 pathway serves as a novel regulator of cellular senescence: Complement of the canonical p53/p21 pathway.
Xu S, Wu W, Huang H, Huang R, Xie L, Su A, Liu S, Zheng R, Yuan Y, Zheng HL, Sun X, Xiong XD, Liu X. Xu S, et al. Aging Cell. 2019 Jun;18(3):e12918. doi: 10.1111/acel.12918. Epub 2019 Mar 7. Aging Cell. 2019. PMID: 30848072 Free PMC article. - Micromanaging aging with miRNAs: new messages from the nuclear envelope.
Ugalde AP, Español Y, López-Otín C. Ugalde AP, et al. Nucleus. 2011 Nov-Dec;2(6):549-55. doi: 10.4161/nucl.2.6.17986. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064465 Review. - Cell autonomous and systemic factors in progeria development.
Osorio FG, Ugalde AP, Mariño G, Puente XS, Freije JM, López-Otín C. Osorio FG, et al. Biochem Soc Trans. 2011 Dec;39(6):1710-4. doi: 10.1042/BST20110677. Biochem Soc Trans. 2011. PMID: 22103512 Review.
Cited by
- MicroRNA-29b/Tet1 regulatory axis epigenetically modulates mesendoderm differentiation in mouse embryonic stem cells.
Tu J, Ng SH, Luk AC, Liao J, Jiang X, Feng B, Lun Mak KK, Rennert OM, Chan WY, Lee TL. Tu J, et al. Nucleic Acids Res. 2015 Sep 18;43(16):7805-22. doi: 10.1093/nar/gkv653. Epub 2015 Jun 30. Nucleic Acids Res. 2015. PMID: 26130713 Free PMC article. - Non-coding RNAs in DNA damage response.
Liu Y, Lu X. Liu Y, et al. Am J Cancer Res. 2012;2(6):658-75. Epub 2012 Nov 20. Am J Cancer Res. 2012. PMID: 23226613 Free PMC article. - Hallmarks of progeroid syndromes: lessons from mice and reprogrammed cells.
Carrero D, Soria-Valles C, López-Otín C. Carrero D, et al. Dis Model Mech. 2016 Jul 1;9(7):719-35. doi: 10.1242/dmm.024711. Dis Model Mech. 2016. PMID: 27482812 Free PMC article. Review. - MicroRNAs and their roles in aging.
Smith-Vikos T, Slack FJ. Smith-Vikos T, et al. J Cell Sci. 2012 Jan 1;125(Pt 1):7-17. doi: 10.1242/jcs.099200. J Cell Sci. 2012. PMID: 22294612 Free PMC article. Review. - Crosstalk between the DNA damage response pathway and microRNAs.
Han C, Wan G, Langley RR, Zhang X, Lu X. Han C, et al. Cell Mol Life Sci. 2012 Sep;69(17):2895-906. doi: 10.1007/s00018-012-0959-8. Epub 2012 Mar 20. Cell Mol Life Sci. 2012. PMID: 22430204 Free PMC article. Review.
References
- Boehm M, Slack F (2005) A developmental timing microRNA and its target regulate life span in C. elegans. Science 310: 1954–1957 - PubMed
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ (2006) Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev 86: 967–1008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous