Integrin β4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF - PubMed (original) (raw)
Integrin β4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF
Jie Fan et al. Ann Biomed Eng. 2011 Aug.
Abstract
Prior studies have indicated that the β4 integrin promotes mammary tumor invasion and metastasis by combining with ErbB2 and amplifying its signaling capacity. However, the effector pathways and cellular functions by which the β4 integrin exerts these effects are incompletely understood. To examine if β4 signaling plays a role during mammary tumor cell adhesion to microvascular endothelium, we have examined ErbB2-transformed mammary tumor cells expressing either a wild-type (WT) or a signaling-defective form of β4 (1355T). We report that WT cells adhere to brain microvascular endothelium in vitro to a significantly larger extent as compared to 1355T cells. Interestingly, integrin β4 signaling does not exert a direct effect on adhesion to the endothelium or the underlying basement membrane. Rather, it enhances ErbB2-dependent expression of VEGF by tumor cells. VEGF in turn disrupts the tight and adherens junctions of endothelial monolayers, enabling the exposure of underlying basement membrane and increasing the adhesion of tumor cells to the intercellular junctions of endothelium. Inhibition of ErbB2 on tumor cells or the VEGFR-2 on endothelial cells suppresses mammary tumor cell adhesion to microvascular endothelium. Our results indicate that β4 signaling regulates VEGF expression by the mammary tumor cells thereby enhancing their adhesion to microvascular endothelium.
Figures
Fig.1
β4 integrin expression of the ErbB2-transformed mouse mammary tumor cells (Neu-β4-WT and Neu-β4-1355T) expressing either wild type β4 (β4-WT) or β4 signaling defect (β4-1355T), and a normal murine mammary gland (NMuMG) epithelial cell line. Cells were lysed and subjected to immunoblotting with anti-β4 and anti-β-actin. * proteolytic fragment of recombinant human β4. • residual endogenous mouse β4.
Fig.2
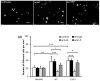
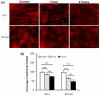
(a) NMuMG, (b) WT and (c) 1355T cell adhesion to bEnd3 cell monolayers. Adhesion was measured 1 hr after seeding under control conditions. The bright spots indicate the adherent fluorescently-labeled cells observed after washing away unattached cells. (d) Comparison of adhesion density (number of adherent cells per unit area of the bEnd3 monolayer) of NMuMG, WT and 1355T cells under control (n=12) and under anti-integrin α6 (n=6) and anti-laminin-5 (n=6) treatment. Anti-mouse IgG was used as a sham control. Cell seeding density of NMuMG, WT and 1355T cells was 2 × 105/ml. The deposit time for both control and treatments was 1 hr. Ten typical fields of 750 μm × 560 μm per run for each cell type were analyzed. *p<0.05, **p<0.01, ***p<0.001.
Fig.3
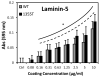
WT and 1355T cell adhesion to laminin-5. WT and 1355T cells of the same passage were placed in the 96-well plate pre-coated with laminin-5 at the graded concentrations. After incubation for 1 hr and removal of unattached cells, the adherent cells in each well were determined by the absorbance as described in the main text. The solid line indicates that there is a significant difference in WT adhesion to ECM proteins compared to the control group (0.01% BSA); the dashed line shows adhesion of 1355T. n=5 for each concentration. *p<0.05 compared to the control (n=8).
Fig.4
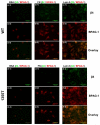
Images of fluorescently-labeled hemidesmosomal marker proteins, β4 integrin (green) and BPAG-1 (red), and the overlay of β4 and BPAG-1 (orange). WT (upper panel) and 1355T (lower panel) tumor cell were allowed to adhere to 10 μg/ml of fibronectin (b, e) and laminin-5 (c, f). Control group adhesion to 0.01% BSA is shown in panels a and d. The amount of β4 integrin and BPAG-1 in tumor cells was quantified based on the pixel numbers of the green labeled area or the red labeled area per μm2, respectively, and related to the total image area, in (g) and (h). The amounts of hemidesmosomes were quantified by the co-localization of β4 integrin and BPAG-1, the orange labeled area, in (i). n=3, *p<0.05, **p<0.01.
Fig.4
Images of fluorescently-labeled hemidesmosomal marker proteins, β4 integrin (green) and BPAG-1 (red), and the overlay of β4 and BPAG-1 (orange). WT (upper panel) and 1355T (lower panel) tumor cell were allowed to adhere to 10 μg/ml of fibronectin (b, e) and laminin-5 (c, f). Control group adhesion to 0.01% BSA is shown in panels a and d. The amount of β4 integrin and BPAG-1 in tumor cells was quantified based on the pixel numbers of the green labeled area or the red labeled area per μm2, respectively, and related to the total image area, in (g) and (h). The amounts of hemidesmosomes were quantified by the co-localization of β4 integrin and BPAG-1, the orange labeled area, in (i). n=3, *p<0.05, **p<0.01.
Fig.5
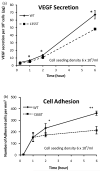
(a) Comparison of temporal VEGF expression profiles of WT and 1355T tumor cells. (b) WT and 1355T tumor cell adhesion to bEnd3 monolayer was assessed at the indicated times. The tumor cell seeding density was 6 × 105/ml. n=3, * p<0.05, **p<0.01.
Fig.6
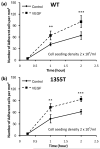
Tumor cell (a) WT and (b) 1355T adhesion as a function of time under control and 1 nM VEGF treatment. The tumor cell seeding density was 2 × 105/ml. n=4, *p<0.05, **p<0.01, ***p<0.001.
Fig. 7
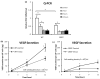
(a) VEGF mRNA levels in WT and 1355T cells. Total RNA was isolated from cells under control and Iressa (10 μM) treatment for the indicated times, and VEGF RNA was quantified by Q-PCR. The data are presented as the ratio of VEGF to GAPDH mRNA (mean±SD) obtained from triplicate samples. The VEGF secretion was quantified by ELISA for (b) WT and (c) 1355T cells under control and under the Iressa treatment at the indicated times. Results are the mean±SD of triplicate samples. Similar results were obtained from two independent experiments. *p<0.05 compared with the control.
Fig.8
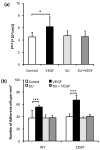
Effects of VEGF and the endothelial VEGF receptor (KDR/Flk-1) inhibitor SU-1498 on (a) Permeability of the bEnd3 monolayer to albumin (n=6) and (b) WT and 1355T tumor cell adhesion. Permeability and adhesion were measured after 1 hr control and VEGF treatment. For inhibition by SU-1498, the bEnd3 monolayer was pretreated with SU-1498 for 30 min. In (b), ten typical fields of 750 μm × 560 μm per run for each cell type in n=6 runs were analyzed. *p=0.045, ***p<0.001.
Fig.9
Histogram of adhesion locations of tumor cells to bEnd3 monolayers under control and VEGF treatment. Typical fluorescent images for (a) WT and (b) 1355T cell adhesion after 1 hr VEGF treatment. Green spots are the tumor cells, red lines are tight junction proteins ZO-1 of the bEnd3 monolayer, and blue spots are bEnd3 endothelial cell nuclei. Summary of adhesion locations for WT (c, e, g) and 1355T (d, f, h) cells under control and 1 nM VEGF treatments for 15 min, 1 hr and 2 hr, respectively. Cell body represents tumor cell adhesion to the body of bEnd3 cells, while Bi-joint and Tri-joint represent tumor cell adhesion to the junctions between two and three (or more) bEnd3 cells (see Fig. 9a). The number of adherent cells was counted from 5 fields of area 240 μm × 180μm in each well. Adhesion for each case was summarized for 2 wells in one experiment. The total number of adherent cells is labeled in the parentheses next to each case. Results are the mean ± SD of three independent experiments (n=3). The p value shown in each figure is from χ2 test for the statistical test between the distribution of the adhesion location under control and that under VEGF treatment. * p<0.05 indicates a significant change in the number of adherent cells at the indicated locations.
Fig.10
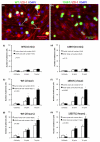
Typical fluorescent images showing the effects of VEGF on bEnd3 endothelial (a) the tight junction protein ZO-1 (upper panel) and the adherens junction protein VE-Cadherin (lower panel) under control (1% BSA) and after 1 hr and 2hr 1nM VEGF treatment. (b) Junction proteins ZO-1 and VE-Cadherin were quantified by the percentage of cell border pixels having a staining intensity higher than the average intensity of the cell cytoplasm (the background) over the total cell border pixels. Ten typical fields of 120 μm × 90 μm per run for n=6 runs were analyzed. **p<0.01, ***p<0.001.
Fig.11
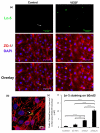
(a) The upper figure is the fluorescent image showing the laminin-5 (green) at the luminal surface of the bEnd3 monolayer, which was stained before permeabilizing the cells; the middle figure shows the junction protein ZO-1 (red) and cell nuclei (blue), which were stained after permeabilizing the cells; and the bottom figure shows the overlay of the upper and middle figures, indicating that laminin-5 was located at the cell-cell junctions. The left panel shows the control result and the right panel shows staining after 1 hr VEGF treatment. The white arrows in the left panel indicate the location of laminin-5. (b) Confocal image of laminin-5 (green), ZO-1 (red) and co-localization of the laminin-5 and ZO-1 at a tri-cellular junction of the bEnd3 monolayer (the region pointed by the white arrow) after 1 hr VEGF treatment. (c) Comparison of the exposed laminin-5 at the luminal surface of the bEnd3 monolayer under control and after 15 min, 1hr and 2 hr VEGF treatment. Data shown is the mean ± SD from 4 runs with ten fields of 240 μm × 180 μm in each run. **p<0.01, ***p<0.001.
Similar articles
- Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis.
Guo W, Pylayeva Y, Pepe A, Yoshioka T, Muller WJ, Inghirami G, Giancotti FG. Guo W, et al. Cell. 2006 Aug 11;126(3):489-502. doi: 10.1016/j.cell.2006.05.047. Cell. 2006. PMID: 16901783 - Co-opted integrin signaling in ErbB2-induced mammary tumor progression.
Carraway KL 3rd, Sweeney C. Carraway KL 3rd, et al. Cancer Cell. 2006 Aug;10(2):93-5. doi: 10.1016/j.ccr.2006.07.015. Cancer Cell. 2006. PMID: 16904607 Review. - Nuclear factor-kappaB enhances ErbB2-induced mammary tumorigenesis and neoangiogenesis in vivo.
Liu M, Ju X, Willmarth NE, Casimiro MC, Ojeifo J, Sakamaki T, Katiyar S, Jiao X, Popov VM, Yu Z, Wu K, Joyce D, Wang C, Pestell RG. Liu M, et al. Am J Pathol. 2009 May;174(5):1910-20. doi: 10.2353/ajpath.2009.080706. Epub 2009 Apr 6. Am J Pathol. 2009. PMID: 19349372 Free PMC article. - Integrin-associated CD151 drives ErbB2-evoked mammary tumor onset and metastasis.
Deng X, Li Q, Hoff J, Novak M, Yang H, Jin H, Erfani SF, Sharma C, Zhou P, Rabinovitz I, Sonnenberg A, Yi Y, Zhou P, Stipp CS, Kaetzel DM, Hemler ME, Yang XH. Deng X, et al. Neoplasia. 2012 Aug;14(8):678-89. doi: 10.1593/neo.12922. Neoplasia. 2012. PMID: 22952421 Free PMC article. - Targeting integrin beta4 for cancer and anti-angiogenic therapy.
Giancotti FG. Giancotti FG. Trends Pharmacol Sci. 2007 Oct;28(10):506-11. doi: 10.1016/j.tips.2007.08.004. Epub 2007 Sep 5. Trends Pharmacol Sci. 2007. PMID: 17822782 Review.
Cited by
- Targeting the tumour vasculature: from vessel destruction to promotion.
Guelfi S, Hodivala-Dilke K, Bergers G. Guelfi S, et al. Nat Rev Cancer. 2024 Oct;24(10):655-675. doi: 10.1038/s41568-024-00736-0. Epub 2024 Aug 29. Nat Rev Cancer. 2024. PMID: 39210063 Review. - Computational analysis of cancer cell adhesion in curved vessels affected by wall shear stress for prediction of metastatic spreading.
Rahmati N, Maftoon N. Rahmati N, et al. Front Bioeng Biotechnol. 2024 May 27;12:1393413. doi: 10.3389/fbioe.2024.1393413. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38860135 Free PMC article. - Heparan Sulfate Modulation Affects Breast Cancer Cell Adhesion and Transmigration across In Vitro Blood-Brain Barrier.
Li Y, Shteyman DB, Hachem Z, Ulay AA, Fan J, Fu BM. Li Y, et al. Cells. 2024 Jan 19;13(2):190. doi: 10.3390/cells13020190. Cells. 2024. PMID: 38275815 Free PMC article. - The Inhibition of Vessel Co-Option as an Emerging Strategy for Cancer Therapy.
Carrera-Aguado I, Marcos-Zazo L, Carrancio-Salán P, Guerra-Paes E, Sánchez-Juanes F, Muñoz-Félix JM. Carrera-Aguado I, et al. Int J Mol Sci. 2024 Jan 11;25(2):921. doi: 10.3390/ijms25020921. Int J Mol Sci. 2024. PMID: 38255995 Free PMC article. Review. - Targeting of endothelial cells in brain tumours.
Duan W, Xia S, Tang M, Lin M, Liu W, Wang Q. Duan W, et al. Clin Transl Med. 2023 Oct;13(10):e1433. doi: 10.1002/ctm2.1433. Clin Transl Med. 2023. PMID: 37830128 Free PMC article. Review.
References
- Abdel-Ghany M, Cheng HC, Elble RC, Pauli BU. Focal adhesion kinase activated by beta(4) integrin ligation to mCLCA1 mediates early metastatic growth. J Biol Chem. 2002;277:34391–34400. - PubMed
- Astolfi A, Landuzzi L, Nicoletti G, De Giovanni C, Croci S, Palladini A, Ferrini S, Iezzi M, Musiani P, Cavallo F, Forni G, Nanni P, Lollini PL. Gene expression analysis of immune-mediated arrest of tumorigenesis in a transgenic mouse model of HER-2/neu-positive basal-like mammary carcinoma. Am J Pathol. 2005;166:1205–1216. - PMC - PubMed
- Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. J Leukoc Biol. 1999;65:299–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA137788/CA/NCI NIH HHS/United States
- P30 CA08748/CA/NCI NIH HHS/United States
- SC1 CA153325/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 CA129023/CA/NCI NIH HHS/United States
- R37 CA058976/CA/NCI NIH HHS/United States
- R01 CA152975/CA/NCI NIH HHS/United States
- P20 CA118861/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous