A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells - PubMed (original) (raw)
A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells
Soumya Jaganathan et al. PLoS One. 2011.
Retraction in
- Retraction: A Functional Nuclear Epidermal Growth Factor Receptor, Src and Stat3 Heteromeric Complex in Pancreatic Cancer Cells.
PLOS ONE Editors. PLOS ONE Editors. PLoS One. 2019 Feb 20;14(2):e0212884. doi: 10.1371/journal.pone.0212884. eCollection 2019. PLoS One. 2019. PMID: 30785961 Free PMC article. No abstract available.
Abstract
Evidence is presented for the nuclear presence of a functional heteromeric complex of epidermal growth factor (EGFR), Src and the Signal Transducer and Activator of Transcription (Stat)3 proteins in pancreatic cancer cells. Stat3 remains nuclear and associated with Src or EGFR, respectively, upon the siRNA knockdown of EGFR or Src, demonstrating the resistance of the complex to the modulation of EGFR or Src alone. Significantly, chromatin immunoprecipitation (ChIP) analyses reveal the nuclear EGFR, Src and Stat3 complex is bound to the c-Myc promoter. The siRNA knockdown of EGFR or Src, or the pharmacological inhibition of Stat3 activity only marginally suppressed c-Myc expression. By contrast, the concurrent modulation of Stat3 and EGFR, or Stat3 and Src, or EGFR and Src strongly suppressed c-Myc expression, demonstrating that the novel nuclear heteromeric complex intricately regulates the c-Myc gene. The prevalence of the transcriptionally functional EGFR, Src, and Stat3 nuclear complex provides an additional and novel mechanism for supporting the pancreatic cancer phenotype and explains in part the insensitivity of pancreatic cancer cells to the inhibition of EGFR, Src or Stat3 alone.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
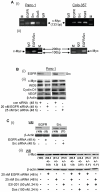
Figure 1. Co-immunoprecipitation with immunoblotting analysis of EGFR, Src and Stat3 association in Panc-1 and Colo-357 cells.
Immunoblotting analyses of immunecomplexes of EGFR (IP:EGFR), Src (IP:Src), and Stat3 (IP:Stat3), or of non-specific IgG non-immunoprecipitate prepared from whole-cell lysates of Panc-1 or Colo-357 cells untransfected (A and B) or transfected with EGFR siRNA, Src siRNA, or control (con) siRNA (C) and probing for Src, Stat3 and EGFR in the absence (A and C) or presence (B) of Stat3 blocking peptide (Stat3 BP), Src blocking peptide (Src BP) or EGFR blocking peptide (EGFR BP). Bands corresponding to proteins in gel are shown; input: except where indicated, represents the immunoblotting for the respective immunoprecipitated protein in the same amount of lysate used in the assay; Data are representative of 3 independent studies.
Figure 2. Co-immunoprecipitation with immunoblotting analysis of EGFR, Src and Stat3 complex in the nucleus and the sub-cellular distribution of EGFR, Src and Stat3.
(A and B) Immunoblotting analyses of immunecomplexes of EGFR (IP:EGFR), Src (IP:Src), Stat3 (IP:Stat3), EGFR/Src (IP:EGFR/IP:Src), or of non-specific IgG non-immuneprecpitate prepared from nuclear extracts of Panc-1 or Colo-357 cells and probing for Stat3, EGFR, Src, or the Tata-binding protein (TBP); and (C), immunoblotting analysis of membrane (mem) and cytosolic (cyto) fractions and of nuclear (nuc) extracts from Panc-1 cells probing for (i) EGFR, (ii) Stat3 and (iii) Src. Bands corresponding to proteins in gel are shown; input: except where indicated, represents the immunoblotting for the respective immunoprecipitated protein in the same amount of nuclear extract used in the assay; IP:EGFR/IP:Src, sequential immunoprecipitation with anti-EGFR and then anti-Src antibody; Data are representative of 3 independent studies.
Figure 3. Co-immunoprecipitation with immunoblotting analysis of the effects of modulation of EGFR, Src and Stat3 on the nuclear EGFR, Src and Stat3 complex.
(A, B, and C) Immunoblotting analyses of immunecomplexes of Stat3 (IP:Stat3), EGFR (IP:EGFR), or Src (IP:Src) prepared from nuclear extracts of Panc-1 cells untransfected or transfected with Src siRNA, EGFR siRNA, or control (con) siRNA (A), or treated with or without the EGFR inhibitor (ZD1839, ZD), Src inhibitor (Dasatinib, Das), or the Stat3 inhibitor (S3I-201) for 1 or 24 h (B), or from nuclear extracts pre-incubated for 2 h with or without 100 µM pY1068, pY1086, or SPI peptide (C) and probing for EGFR, Src, Stat3; or (D) immunoblotting analysis of nuclear extracts prepared from Panc-1 cells treated or untreated with phenylarsine oxide (PAO) and probing for Src, Stat3, EGFR. Bands corresponding to proteins in gel are shown; input: except where indicated, represents the immunoblotting for the respective immunoprecipitated protein in the same amount of lysate or nuclear extract used in the assay; Data are representative of 3 independent studies.
Figure 4. Studies of protein complex and protein binding partners using the Detection and Analysis through Nanoparticle Sizing technology.
(A) Kinetic binding assay of EGFR-gold nanoparticle (GNP) probe (or mouse IgG1-GNP probe as negative control) binding to (i) EGFR protein and its complex from Panc-1 nuclear extracts, and the (ii) inhibitory effect of the mouse monoclonal anti-EGFR antibody on the EGFR-GNP probe binding to the EGFR protein; and (B) Protein complex binding partner analysis whereby the polyclonal anti-Stat3, anti-Src or anti-EGFR antibody or the non-specific rabbit IgG (negative control) is added to the assay solution prepared from the (i) non-specific mouse IgG1-GNP probe (negative control), or (ii) anti-EGFR-GNP probe; Data are representative of 4 independent studies.
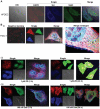
Figure 5. Immunofluorescence with laser-scanning confocal microscopy of EGFR, Src and Stat3 association in HPDEC or Panc-1 cells.
Cultured normal human pancreatic duct epithelial cells (HPDEC) (A) or pancreatic cancer, Panc-1 cells (B) were fixed, stained with primary antibodies against EGFR, Src and Stat3 and their corresponding secondary antibodies, ALexaFLuor405 (goat anti-mouse, EGFR, red), AlexaFluor488 (donkey anti-rabbit, Src, blue) and AlexaFluor546 (goat anti-rat, Stat3, green) and analyzed by laser-scanning confocal microscopy for localization (single) and colocalization (merge) studies of EGFR (red), Src (blue) and Stat3 (green) and the effects of treatment (i) without or (ii) with ZD1839 (ZD) or (iii) Dasatinib (Das) for the indicated times. Confocal images were collected using Leica TCS SP5 microscopes; Cyan, magenta, yellow and white/pale yellow arrows denote merged colors; single, one color capture, merged, three-color capture. Data are representative of 3 independent studies.
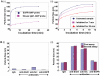
Figure 6. Chromatin immunoprecipitation assay and Western blotting analysis of c-Myc, iNOS, Cyclin D1, and VEGF expression in Panc-1 and Colo-357 cells.
(A), Agarose gel electrophoresis of the Polymerase Chain Reaction (PCR)-amplified c-Myc gene fragment from the chromatin DNA precipitated with antibody against EGFR, Src, or Stat3, or with the non-specific IgG; and (B and C), Immunoblotting analysis of whole-cell lysates probing for EGFR or Src (B(i) and C(i)) or c-Myc, iNOS, Cyclin D1 or VEGF (B(ii) and C(ii)), and the effects of siRNA knockdown of EGFR (EGFR siRNA), Src (Src siRNA) or control (con) siRNA, or S3I-201 or Das). Bands corresponding to proteins or c-Myc gene in gel are shown; M, molecular weight marker, EGFR/Src, sequential immunoprecipitation with anti-EGFR and then anti-Src antibody. Data are representative of 3 independent studies, and values are mean and s.d of 3 independent studies; *_p_-<0.01.
Similar articles
- Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src.
Jaganathan S, Yue P, Turkson J. Jaganathan S, et al. J Pharmacol Exp Ther. 2010 May;333(2):373-81. doi: 10.1124/jpet.109.162669. Epub 2010 Jan 25. J Pharmacol Exp Ther. 2010. PMID: 20100905 Free PMC article. - Signal transducer and activator of transcription 5b, c-Src, and epidermal growth factor receptor signaling play integral roles in estrogen-stimulated proliferation of estrogen receptor-positive breast cancer cells.
Fox EM, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM. Fox EM, et al. Mol Endocrinol. 2008 Aug;22(8):1781-96. doi: 10.1210/me.2007-0419. Epub 2008 Jun 11. Mol Endocrinol. 2008. PMID: 18550772 Free PMC article. - A novel nuclear Src and p300 signaling axis controls migratory and invasive behavior in pancreatic cancer.
Paladino D, Yue P, Furuya H, Acoba J, Rosser CJ, Turkson J. Paladino D, et al. Oncotarget. 2016 Feb 9;7(6):7253-67. doi: 10.18632/oncotarget.6635. Oncotarget. 2016. PMID: 26695438 Free PMC article. - Nuclear Dynamics and Chromatin Structure: Implications for Pancreatic Cancer.
Flores LF, Tader BR, Tolosa EJ, Sigafoos AN, Marks DL, Fernandez-Zapico ME. Flores LF, et al. Cells. 2021 Oct 1;10(10):2624. doi: 10.3390/cells10102624. Cells. 2021. PMID: 34685604 Free PMC article. Review.
Cited by
- Developing a nanoparticle test for prostate cancer scoring.
Huo Q, Litherland SA, Sullivan S, Hallquist H, Decker DA, Rivera-Ramirez I. Huo Q, et al. J Transl Med. 2012 Mar 9;10:44. doi: 10.1186/1479-5876-10-44. J Transl Med. 2012. PMID: 22404986 Free PMC article. - Prediction of oncogenic interactions and cancer-related signaling networks based on network topology.
Acencio ML, Bovolenta LA, Camilo E, Lemke N. Acencio ML, et al. PLoS One. 2013 Oct 25;8(10):e77521. doi: 10.1371/journal.pone.0077521. eCollection 2013. PLoS One. 2013. PMID: 24204854 Free PMC article. - STAT3 Interactors as Potential Therapeutic Targets for Cancer Treatment.
Laudisi F, Cherubini F, Monteleone G, Stolfi C. Laudisi F, et al. Int J Mol Sci. 2018 Jun 16;19(6):1787. doi: 10.3390/ijms19061787. Int J Mol Sci. 2018. PMID: 29914167 Free PMC article. Review. - Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo.
Lee JG, Wu R. Lee JG, et al. Neoplasia. 2015 Feb;17(2):190-200. doi: 10.1016/j.neo.2014.12.008. Neoplasia. 2015. PMID: 25748238 Free PMC article. - Access to the nucleus and functional association with c-Myc is required for the full oncogenic potential of ΔEGFR/EGFRvIII.
Gururaj AE, Gibson L, Panchabhai S, Bai M, Manyam G, Lu Y, Latha K, Rojas ML, Hwang Y, Liang S, Bogler O. Gururaj AE, et al. J Biol Chem. 2013 Feb 1;288(5):3428-38. doi: 10.1074/jbc.M112.399352. Epub 2012 Dec 17. J Biol Chem. 2013. PMID: 23250739 Free PMC article.
References
- Ulrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. - PubMed
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. - PubMed
- Darnell JE. Validating Stat3 in cancer therapy. Nat Med. 2005;11:595–596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous