Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway - PubMed (original) (raw)
Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway
Haitao Luo et al. Food Chem. 2011.
Abstract
Ovarian cancer is a significant malignancy for women in the western world, and its death rate has remained unchanged over the past 50 years, leaving room for proper chemoprevention. Kaempferol is a natural flavonoid widely distributed in fruits and vegetables, and epidemiological studies have found a negative correlation between kaempferol consumption and ovarian cancer risk. To understand the mechanism behind this negative correlation, we investigated kaempferol's ability to induce apoptosis in A2780/CP70, A2780/wt, and OVCAR-3 ovarian cancer cell lines. Kaempferol inhibited cell proliferation but did not cause necrosis in all 3 cell lines. For the apoptosis, caspase 3/7 levels were induced in a concentration-dependent manner by kaempferol treatment, with A2780/wt cells being the most responsive. This induction can be diminished by pre-treatment with a caspase-9 inhibitor, indicating an intrinsic apoptosis pathway. Western blot analysis revealed that protein levels of Bcl-x(L) were decreased in ovarian cancer cells, while p53, Bad, and Bax proteins were up-regulated by kaempferol treatment. Our data indicate that kaempferol induces apoptosis in ovarian cancer cells through regulating pro-apoptotic and anti-apoptotic protein expressions in the intrinsic apoptosis pathways, and is a good candidate for the chemoprevention of ovarian cancers in humans. Further studies in animal models and clinical trials are therefore warranted.
Figures
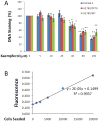
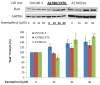
Figure 1. Kaempferol inhibits proliferation of ovarian cancer cells at 40-uM or higher concentrations
A. OVCAR-3, A2780/wt, and A2780/CP70 cells were seeded into 96-well plates at 2000 cells/well and incubated overnight before treatment with various concentration of kaempferol in triplicate for 24 hours. After removing medium, the plate was frozen at -20°C for 30 minutes, thawed at room temperature, and cells were lysed with 200 μl 1x CyQUANT cell lysis buffer containing 5X SYBR Green I dye. After 5 minutes incubation at room temperature, cell lysates (50 μl) were transferred to PCR strip tubes and the fluorescence were measured at 90 °C in a real-time PCR instrument. Data represent Means ± SE from 3 independent experiments. * p<0.05, **p<0.01 as compared to control. B. A standard curve was generated by seeding different numbers of OVCAR-3 cells in 96-well plate, incubating them overnight, and measuring DNA abundance as described above in A.
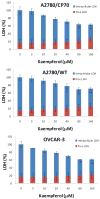
Figure 2. Kaempferol has no cytotoxicity on ovarian cancer cells
Ovarian cancer cells were seeded in 96-well plate at 5000 cells/well and incubated overnight before treatment with kaempferol for 24 hours. Culture medium was sampled for measurement of free LDH levels. Cell culture were then added with Lysis Solution and incubated at 37°C for 1 hour before being sampled again to measure total LDH levels. LDH levels were analyzed with a CytoTox 96 Non-Radioactive Cytotoxicity Assay from Promega. None-cell culture medium was assayed to the control background, and contained LDH levels were derived by subtracting free LDH levels from total LDH levels. All LDH levels within a single experiment were standardized to the total LDH level of control for statistical analysis. Data represent Mean ± SE from 2 or 3 independent experiments.
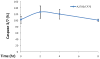
Figure 3. Time course of kaempferol-induced apoptosis in A2780/CP70 ovarian cancer cells
A2780/CP70 ovarian cancer cells were seeded in 96-well plates at 10,000 cells/well and incubated overnight. The cells were treated with 0- or 80-μM kaempferol in triplicates for 0, 2, 4, or 8 hours. No-cell wells were included for background correction. At each time point, a plate was removed of culture medium and frozen in -80 °C until final analysis. Cells were thawed, lyzed with Passive Lysis Bufer, and analyzed for caspase 3/7 activities with a Caspase-Glo 3/7 Assay and total protein levels with a BCA assay. Caspase 3/7 activities were normalized by total protein levels, and the levels of kaempferol-treated cells were expressed as percentages of the controls for statistics. Data represent Means ± SE from 2 independent experiments.
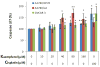
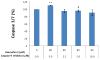
Figure 4. Kaempferol induces apoptosis in ovarian cancer cells
Ovarian cancer cells were seeded in two 96-well plates at 10,000 cells/well and incubated overnight before treatment with kaempferol in triplicate for 2 hours. Plates were removed of culture medium and frozen at -80 °C. For apoptosis analysis, cells were thawed and lyzed with Passive Lysis Buffer, and measured for caspase 3/7 activities and total protein levels. No-cell wells were included for background correction. Caspase 3/7 activities were adjusted by total protein levels and expressed as percentages of the controls. Data represent Means ± SE from 6 or 7 independent experiments. *p<0.05, **p<0.01 as compared to control.
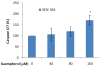
Figure 5. Kaempferol induces apoptosis in immortalized normal ovarian cells only at high concentration
Immotalized normal ovarian epithelial cell line IOSE 364 were seeded in 96-well plates at 10,000 cells/well and incubated overnight. The cells were treated with various concentrations of kaempferol in triplicates for 2 hours and no-cell wells were included for background correction. Plates were then removed of culture medium and frozen at -80 °C. For apoptosis analysis, cells were thawed and lyzed with Passive Lysis Buffer, and measured for caspase 3/7 activities and total protein levels. Caspase 3/7 activities were normalized by total protein levels and expressed as percentages of controls. Data represent Means ± SE from 3 independent experiments. *p<0.05 as compared to control.
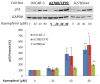
Figure 6. Kaempferol induces apoptosis through intrinsic pathway in OVCAR-3 ovarian cancer cells
OVCAR-3 cells were seeded at 10,000 cells/well in 96-well plates and incubated overnight. The cells were pre-treated with various concentrations of a caspase-9 inhibitor (Z-LEHDFMK) or negative control (Z-FA-FMK) in triplicate for 24 hours, and treated with 0 or 80 μM of kaempferol for 2 hours. For the caspase 3/7 assay, 100 μl freshly prepared reagent was added to each well, incubated for 1 hour at RT, and 60 μl of the reaction mixture was transferred to glass tubes to measure luminescence. For cell number assay, 100 μl of freshly prepared AqueousOne reagent was added, incubated for 1 hour at RT, and measured at OD 560 with a microplate reader. Non-cell wells were included to measure background values for both assays. Caspase 3/7 values were adjusted by cell number values. Data represent Means ± SE from 2 independent experiments. **p<0.01 as compared to control. #p<0.05 as compared to the kaempferol treated control.
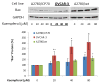
Figure 7. Kaempferol increases “p53” protein levels in ovarian cancer cells
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “p53” protein in western blots. Images shown are typical western blot bands for “p53” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “p53” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
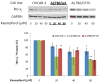
Figure 8. Kaempferol increases “Bad” protein levels in ovarian cancer cells
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “Bad” protein in western blots. Images shown are typical western blot bands for “Bad” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “Bad” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
Figure 9. Kaempferol increases “Bax” protein levels in ovarian cancer cells
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “Bax” protein in western blots. Images shown are typical western blot bands for “Bax” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “Bax” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 2-3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
Figure 10. Kaempferol decreases “Bcl-xL” protein levels in ovarian cancer cells
Ovarian cancer cells were seeded in 60-mm dishes, incubated overnight, and treated with kaempferol for 24 hours. The cells were harvested, subjected to SDS-PAGE, and probed with antibodies against human “Bcl-xL” protein in western blots. Images shown are typical western blot bands for “BcL-xL” protein and their corresponding “GAPDH” bands. Protein bands were quantified with “ImageJ” software and “Bcl-xL” protein levels were adjusted by “GAPDH” levels. Data represent Means ± SE from 2-3 independent experiments. *p<0.05 as compared to control; **p<0.01 as compared to control.
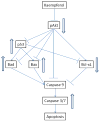
Figure 11. Proposed mechanism/pathways for kaempferol-induced apoptosis in ovarian cancer cells
Kaempferol treatment in ovarian cancer cells inhibits Akt phosphorylation, which in turn inhibits expression of Bcl-xL gene but up-regulates expression of p53, Bad, and Bax genes, leading to an imbalance between pro-apoptosis and anti-apoptotic factors and in favour of activated Caspase 3/7 and intrinsic apoptosis.
Similar articles
- Kaempferol Induces G2/M Cell Cycle Arrest via Checkpoint Kinase 2 and Promotes Apoptosis via Death Receptors in Human Ovarian Carcinoma A2780/CP70 Cells.
Gao Y, Yin J, Rankin GO, Chen YC. Gao Y, et al. Molecules. 2018 May 5;23(5):1095. doi: 10.3390/molecules23051095. Molecules. 2018. PMID: 29734760 Free PMC article. - Galangin, a Flavonoid from Lesser Galangal, Induced Apoptosis via p53-Dependent Pathway in Ovarian Cancer Cells.
Huang H, Chen AY, Ye X, Guan R, Rankin GO, Chen YC. Huang H, et al. Molecules. 2020 Mar 30;25(7):1579. doi: 10.3390/molecules25071579. Molecules. 2020. PMID: 32235536 Free PMC article. - Kaempferol enhances cisplatin's effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc.
Luo H, Daddysman MK, Rankin GO, Jiang BH, Chen YC. Luo H, et al. Cancer Cell Int. 2010 May 11;10:16. doi: 10.1186/1475-2867-10-16. Cancer Cell Int. 2010. PMID: 20459793 Free PMC article. - The Role of Phytonutrient Kaempferol in the Prevention of Gastrointestinal Cancers: Recent Trends and Future Perspectives.
Singh T, Sharma D, Sharma R, Tuli HS, Haque S, Ramniwas S, Mathkor DM, Yadav V. Singh T, et al. Cancers (Basel). 2024 Apr 27;16(9):1711. doi: 10.3390/cancers16091711. Cancers (Basel). 2024. PMID: 38730663 Free PMC article. Review. - A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention.
Chen AY, Chen YC. Chen AY, et al. Food Chem. 2013 Jun 15;138(4):2099-107. doi: 10.1016/j.foodchem.2012.11.139. Epub 2012 Dec 28. Food Chem. 2013. PMID: 23497863 Free PMC article. Review.
Cited by
- Network Pharmacology and Molecular Docking Analysis on Molecular Targets and Mechanisms of Bushen Hugu Decoction in the Treatment of Malignant Tumor Bone Metastases.
Sang T, Zhang T, Wang J, Zheng Y. Sang T, et al. Biomed Res Int. 2022 Nov 16;2022:2055900. doi: 10.1155/2022/2055900. eCollection 2022. Biomed Res Int. 2022. PMID: 36440359 Free PMC article. - Venus Flytrap (Dionaea muscipula Solander ex Ellis) Contains Powerful Compounds that Prevent and Cure Cancer.
Gaascht F, Dicato M, Diederich M. Gaascht F, et al. Front Oncol. 2013 Aug 20;3:202. doi: 10.3389/fonc.2013.00202. eCollection 2013. Front Oncol. 2013. PMID: 23971004 Free PMC article. - Kaempferol mitigates Endoplasmic Reticulum Stress Induced Cell Death by targeting caspase 3/7.
Abdullah A, Ravanan P. Abdullah A, et al. Sci Rep. 2018 Feb 1;8(1):2189. doi: 10.1038/s41598-018-20499-7. Sci Rep. 2018. PMID: 29391535 Free PMC article. - p53 Enhances Artemisia annua L. Polyphenols-Induced Cell Death Through Upregulation of p53-Dependent Targets and Cleavage of PARP1 and Lamin A/C in HCT116 Colorectal Cancer Cells.
Jung EJ, Lee WS, Paramanantham A, Kim HJ, Shin SC, Kim GS, Jung JM, Ryu CH, Hong SC, Chung KH, Kim CW. Jung EJ, et al. Int J Mol Sci. 2020 Dec 7;21(23):9315. doi: 10.3390/ijms21239315. Int J Mol Sci. 2020. PMID: 33297377 Free PMC article. - Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability.
Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. Wang H, et al. Anticancer Agents Med Chem. 2012 Dec;12(10):1281-305. doi: 10.2174/187152012803833026. Anticancer Agents Med Chem. 2012. PMID: 22583408 Free PMC article. Review.
References
- Asselin E, Mills GB, Tsang BK. XIAP Regulates Akt Activity and Caspase-3-dependent Cleavage during Cisplatin-induced Apoptosis in Human Ovarian Epithelial Cancer Cells. Cancer Research. 2001;61:1862–1868. - PubMed
- Banks E. The Epidemiology of Ovarian Cancer. In: Bartlett JMS, editor. Ovarian Cancer Methods and Protocols. Totowa: Humana Press; 2000. pp. 3–11.
- Gates MA, Tworoger SS, Hecht JL, Vivo ID, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. International Journal of Cancer. 2007;121(10):2225–2232. - PubMed
- Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF, Li KH, Chen PY, Chung JG, Yang JS. Kaempferol Induced Apoptosis via Endoplasmic Reticulum Stress and Mitochondria-dependent Pathway in Human Osteosarcoma U-2 OS Cells. Mol Nutr Food Res. 2010;54:1–11. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous