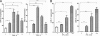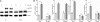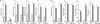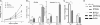MicroRNA miR-21 regulates the metastatic behavior of B16 melanoma cells - PubMed (original) (raw)
MicroRNA miR-21 regulates the metastatic behavior of B16 melanoma cells
Chuan He Yang et al. J Biol Chem. 2011.
Abstract
MicroRNA-21 (miR-21) is overexpressed in many human tumors and has been linked to various cellular processes altered in cancer. miR-21 is also up-regulated by a number of inflammatory agents, including IFN, which is of particular interest considering the close relationship between inflammation and cancer. Because miR-21 appears to be overexpressed in human melanoma, we examined the role of miR-21 in cancer development and metastasis in B16 mouse melanoma cells. We found that miR-21 is a member of an IFN-induced miRNA subset that requires STAT3 activation. To characterize the role of miR-21 in melanoma behavior, we transduced B16 cells with lentivirus encoding a miR-21 antagomir and isolated miR-21 knockdown B16 cells. miR-21 knockdown or IFN treatment alone inhibited B16 cell proliferation and migration in vitro, and in combination they had an enhanced effect. Moreover, miR-21 knockdown sensitized B16 cells to IFN-induced apoptosis. In B16 cells miR-21 targeted tumor suppressor (PTEN and PDCD4) and antiproliferative (BTG2) proteins. To characterize the role of miR-21 in vivo, empty vector- and antagomiR-21-transduced B16 melanoma cells were injected via tail vein into syngeneic C57BL/6 mice. Although empty vector-transduced B16 cells produced large lung metastases, miR-21 knockdown cells only formed small lung lesions. Importantly, miR-21 knockdown tumor-bearing mice exhibited prolonged survival compared with empty vector tumor-bearing mice. Thus, miR-21 regulates the metastatic behavior of B16 melanoma cells by promoting cell proliferation, survival, and migration/invasion as well as by suppressing IFN action, providing important new insights into the role of miR-21 in melanoma.
Figures
FIGURE 1.
Characterization of IFN-induced miR-21 expression in B16 melanoma cells in vitro. B16 cells were treated with IFN at 1,000 units/ml for the indicated times (A) or at the indicated doses for 6 h (B), and miR-21 was quantified by qPCR. IFN-induced ISG15 induction is shown for comparison (n = 3). Error bars, S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FIGURE 2.
Role of STAT3 in IFN-induced miRNA expression in B16 melanoma cells in vitro. B16 cells transduced with EV or F705-STAT3 were treated with IFN (1,000 units/ml) and cell lysates were prepared and immunoblotted as indicated (A), or after 6 h of IFN treatment the indicated miRNAs were quantified by qPCR (n = 3) (B). Error bars, S.D. **, p < 0.01. NS, not significant.
FIGURE 3.
Effects of miR-21KD on miRNA expression and IFN-induced gene expression in B16 cells in vitro. B16 cells transduced with anti-miR-21 lentivirus (miR-21KD) or with empty vector (EV) were treated with IFN (1,000 units/ml), and total RNA assayed for miRNA expression (A) or IFN-induced gene expression by qPCR (n = 3) (B). Error bars, S.D. **, p < 0.01; ***, p < 0.001.
FIGURE 4.
Effects of miR-21KD on B16 melanoma cell proliferation, apoptosis, migration, and protein expression in vitro. A–C, EV and miR-21KD B16 cells were treated with IFN (1,000 units/ml), and at daily intervals cell numbers were determined in a Coulter Counter (n = 3) (A), or after 48 h apoptosis was determined by cell death ELISA or by Annexin V-staining (n = 3) (B), or after 24 h cell migration was determined by transwell migration assays (n = 3) (C). D, EV and miR-21KD B16 cells were lysed, and the expression of key target proteins was determined by immunoblotting with anti-PTEN, PDCD4, BTG2, and SPRY2. Error bars, S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
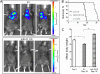
FIGURE 5.
Decreased metastatic potential of miR-21KD B16 melanoma cells in vivo. Mice were injected into the tail vein with 106 EV or miR-21KD B16 cells. A, bioluminescent imaging of mice injected with luciferin performed at day 16 (n = 8). B, Kaplan-Meier analysis of survival data (n = 8). C, mean body weight of mice at day 18 (n = 8). Error bars, S.D. *, p < 0.05; **, p < 0.01.
Similar articles
- Long noncoding RNA ZFAS1 promotes tumorigenesis through regulation of miR-150-5p/RAB9A in melanoma.
Liang L, Zhang Z, Qin X, Gao Y, Zhao P, Liu J, Zeng W. Liang L, et al. Melanoma Res. 2019 Dec;29(6):569-581. doi: 10.1097/CMR.0000000000000595. Melanoma Res. 2019. PMID: 30889053 - Hydroxyurea regulates the development and survival of B16 Melanoma Cells by upregulating MiR-7013-3p.
Huang YJ, Gao Y, Wang CJ, Han DX, Zheng Y, Wang WH, Jiang H, Yuan B, Zhang JB. Huang YJ, et al. Int J Med Sci. 2021 Mar 3;18(8):1877-1885. doi: 10.7150/ijms.52177. eCollection 2021. Int J Med Sci. 2021. PMID: 33746605 Free PMC article. - microRNA-7-5p inhibits melanoma cell proliferation and metastasis by suppressing RelA/NF-κB.
Giles KM, Brown RA, Ganda C, Podgorny MJ, Candy PA, Wintle LC, Richardson KL, Kalinowski FC, Stuart LM, Epis MR, Haass NK, Herlyn M, Leedman PJ. Giles KM, et al. Oncotarget. 2016 May 31;7(22):31663-80. doi: 10.18632/oncotarget.9421. Oncotarget. 2016. PMID: 27203220 Free PMC article. - miR in melanoma development: miRNAs and acquired hallmarks of cancer in melanoma.
Bennett PE, Bemis L, Norris DA, Shellman YG. Bennett PE, et al. Physiol Genomics. 2013 Nov 15;45(22):1049-59. doi: 10.1152/physiolgenomics.00116.2013. Epub 2013 Sep 17. Physiol Genomics. 2013. PMID: 24046283 Free PMC article. Review. - Searching for the 'melano-miRs': miR-214 drives melanoma metastasis.
Bar-Eli M. Bar-Eli M. EMBO J. 2011 May 18;30(10):1880-1. doi: 10.1038/emboj.2011.132. EMBO J. 2011. PMID: 21593728 Free PMC article. Review.
Cited by
- MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN.
Liu ZL, Wang H, Liu J, Wang ZX. Liu ZL, et al. Mol Cell Biochem. 2013 Jan;372(1-2):35-45. doi: 10.1007/s11010-012-1443-3. Epub 2012 Sep 6. Mol Cell Biochem. 2013. PMID: 22956424 - Nanoparticle delivery of miR-21-3p sensitizes melanoma to anti-PD-1 immunotherapy by promoting ferroptosis.
Guo W, Wu Z, Chen J, Guo S, You W, Wang S, Ma J, Wang H, Wang X, Wang H, Ma J, Yang Y, Tian Y, Shi Q, Gao T, Yi X, Li C. Guo W, et al. J Immunother Cancer. 2022 Jun;10(6):e004381. doi: 10.1136/jitc-2021-004381. J Immunother Cancer. 2022. PMID: 35738798 Free PMC article. - microRNA-10b is a prognostic biomarker for melanoma.
Saldanha G, Elshaw S, Sachs P, Alharbi H, Shah P, Jothi A, Pringle JH. Saldanha G, et al. Mod Pathol. 2016 Feb;29(2):112-21. doi: 10.1038/modpathol.2015.149. Epub 2016 Jan 8. Mod Pathol. 2016. PMID: 26743475 - The curcumin analog EF24 targets NF-κB and miRNA-21, and has potent anticancer activity in vitro and in vivo.
Yang CH, Yue J, Sims M, Pfeffer LM. Yang CH, et al. PLoS One. 2013 Aug 7;8(8):e71130. doi: 10.1371/journal.pone.0071130. Print 2013. PLoS One. 2013. PMID: 23940701 Free PMC article. - Prognostic role of microRNA-21 in colorectal cancer: a meta-analysis.
Xia X, Yang B, Zhai X, Liu X, Shen K, Wu Z, Cai J. Xia X, et al. PLoS One. 2013 Nov 12;8(11):e80426. doi: 10.1371/journal.pone.0080426. eCollection 2013. PLoS One. 2013. PMID: 24265822 Free PMC article.
References
- Bartel D. P. (2004) Cell 116, 281–297 - PubMed
- Calin G. A., Croce C. M. (2006) Nat. Rev. Cancer 6, 857–866 - PubMed
- Si M. L., Zhu S., Wu H., Lu Z., Wu F., Mo Y. Y. (2007) Oncogene 26, 2799–2803 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous