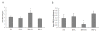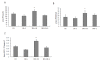Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway - PubMed (original) (raw)
Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway
Jia Y Guo et al. J Biomed Sci. 2011.
Abstract
Background: Ischemic postconditioning (IPO) has been demonstrated to attenuate ischemia/reperfusion (I/R) injury in the heart and brain, its roles to liver remain to be defined. The study was undertaken to determine if IPO would attenuate liver warm I/R injury and its protective mechanism.
Methods: Mice were divided into sham, I/R, IPO+I/R (occlusing the porta hepatis for 60 min, then treated for three cycles of 10 sec brief reperfusion consecutively, followed by a persistent reperfusion); L-NAME+ sham (L-NAME, 16 mg/kg, i.v., 5 min before repefusion); L-NAME+I/R; and L-NAME+ IPO. Blood flow of caudate and left lobe of the liver was blocked. Functional and morphologic changes of livers were evaluated. Contents of nitric oxide, eNOS and iNOS in serum were assayed. Concentration of eNOS, iNOS, malondialdehyde (MDA) and activity of superoxide dismutase (SOD) in hepatic tissue were also measured. Expressions of Akt, p-Akt and HIF-1α protein were determined by western blot. Expressions of TNF-α and ICAM-1 were measured by immunohistochemistry and RT-PCR.
Results: IPO attenuated the dramatically functional and morphological injuries. The levels of ALT was significantly reduced in IPO+I/R group (p < 0.05). Contents of nitric oxide and eNOS in serum were increased in the IPO+I/R group (p < 0.05). IPO also up-regulated the concentration of eNOS, activity of SOD in hepatic tissue (p < 0.05), while reduced the concentration of MDA (p < 0.05). Moreover, protein expressions of HIF-1α and p-Akt were markedly enhanced in IPO+I/R group. Protein and mRNA expression of TNF-α and ICAM-1 were markedly suppressed by IPO (p < 0.05). These protective effects of IPO could be abolished by L-NAME.
Conclusions: We found that IPO increased the content of NO and attenuated the overproduction of ROS and I/R-induced inflammation. Increased NO contents may contribute to increasing HIF-1α level, and HIF-1α and NO would simultaneously protect liver from I/R injury. These findings suggested IPO may have the therapeutic potential through Akt-eNOS-NO-HIF pathway for the better management of liver I/R injury.
Figures
Figure 1
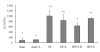
ALT levels after reperfusion. After 60 minutes of ischemia and 2 hours of reperfusion, serum levels of ALT were determined. Compared with sham-operated control mice, I/R mice showed significant increases in ALT. The post-treatment of IPO significantly reduced all serum levels of ALT compared to I/R group. "+L" means "+L-NAME". For all groups, n = 8. * p < 0.05 compared to sham group. † p < 0.05 compared to IPO+I/R group.
Figure 2
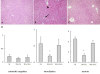
Hepatic histological changes in mice subjected to I/R. (A): sham, (B): I/R, (C): IPO+I/R. Hematoxylin-eosin-stained liver sections from animals undergoing 60 minutes ischemia and 2 hours following reperfusion (Original magnification: ×200). Decreased hepatic necrosis is seen in the IPO+I/R group compared to the nontreated I/R group. Images are representative liver sections from eight mice per group. Black arrow shows the infiltrated neutrophils and black arrow head shows hepatic cellular necrosis in Figure 2B. (D): Histological scores for sinusoidal congestion, cytoplasmic vacuolization, and hepatocyte necrosis were obtained via analysis of hematoxylin-eosin staining. Data are expressed as the mean ± SD of 8 animals per group. * p < 0.05 compared with I/R group.
Figure 3
Effects of IPO on SOD (A) and MDA (B) levels in liver tissues. To assess the effect of IPO on oxidative stress after liver I/R, MDA and activity of SOD were measured. Hepatic 60 minutes ischemia and 2 hours of reperfusion caused substantial increase in liver MDA levels and marked decrease in liver SOD activity compared with IPO+I/R group. For all groups, n = 8. *Significant at p < 0.05 when compared with I/R group.
Figure 4
Effects of IPO on NO in serum (A), eNOS in serum (B) and in liver tissues (C). To determine whether IPO have protective role through NO-mediated production, the contents of NO and NOS were detected. Compared to I/R group, IPO post-treatment markedly induced NO and eNOS production in serum and in liver tissues. For all groups, n = 8. * p < 0.05 compared with I/R group.
Figure 5
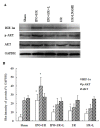
Expression of HIF-1alpha, p-Akt and Akt by Western blot. The expression of HIF-1alpha, p-Akt and Akt were detected in liver tissues by western blot analysis. The blot shown is representative of three different experiments with similar results (A). Lain 1-5: sham; IPO+I/R; IPO+I/R+L-NAME; I/R; I/R+L-NAME. The expression of the housekeeping gene, glyceraldehydes 3-phosphate dehydrogenase (GAPDH), served as a control. The expression of HIF-1alpha, and p-Akt were significantly higher in the liver tissues with IPO+I/R group than I/R group, and the signals were decreased in liver tissues with L-NAME (16 mg/kg) pre-treatment. HIF-1alpha, p-Akt and Akt proteins were calculated by densitometry relative to GAPDH, and the results were expressed as ratios after normalization at 100% of the control (B). Data are mean ± SD from three separate experiments. * p < 0.05 compared with other groups. † p < 0.05 compared to sham group.
Figure 6
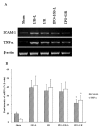
RT-PCR product of TNF-α and ICAM-1 using template RNA extracted from 4 h post-ischemic liver tissues (A). IPO significantly abrogated liver warm I/R-induced increases in TNF-α and ICAM-1 mRNA expression. Lain 1-5: sham; I/R+L-NAME; I/R; IPO+I/R+L-NAME; IPO+I/R. Representative experiments of three are shown in each case. The mRNA band intensities of TNF-α and ICAM-1 in sham, I/R+L-NAME, I/R, IPO+I/R+L-NAME, IPO+I/R groups were compared as indicated (B). (n = 8). Data are mean ± SD from three separate experiments. * p < 0.05 compared with other groups.
Figure 7
Immunohistochemical assay of TNF-α (A, B, C) and ICAM-1(D, E, F) on 4 h post-ischemic liver tissue. In the IPO+I/R group, hepatic I/R-induced increases in TNF-α and ICAM-1 expression were dramatically suppressed. (A, D): sham group, (B, E): I/R group, (C, F): IPO+I/R group. Original magnification: ×400.
Similar articles
- Rb1 postconditioning attenuates liver warm ischemia-reperfusion injury through ROS-NO-HIF pathway.
Guo Y, Yang T, Lu J, Li S, Wan L, Long D, Li Q, Feng L, Li Y. Guo Y, et al. Life Sci. 2011 Mar 28;88(13-14):598-605. doi: 10.1016/j.lfs.2011.01.022. Epub 2011 Feb 12. Life Sci. 2011. PMID: 21300075 - Ischemic preconditioning reduces endoplasmic reticulum stress and upregulates hypoxia inducible factor-1α in ischemic kidney: the role of nitric oxide.
Mahfoudh-Boussaid A, Zaouali MA, Hadj-Ayed K, Miled AH, Saidane-Mosbahi D, Rosello-Catafau J, Ben Abdennebi H. Mahfoudh-Boussaid A, et al. J Biomed Sci. 2012 Jan 17;19(1):7. doi: 10.1186/1423-0127-19-7. J Biomed Sci. 2012. PMID: 22252226 Free PMC article. - Protective effect of nitric oxide on hepatopulmonary syndrome from ischemia-reperfusion injury.
Diao TJ, Chen X, Deng LH, Chen HX, Liang Y, Zhao XD, Wang QH, Yuan WS, Gao BC, Ye Y. Diao TJ, et al. World J Gastroenterol. 2012 Jul 7;18(25):3310-6. doi: 10.3748/wjg.v18.i25.3310. World J Gastroenterol. 2012. PMID: 22783057 Free PMC article. - Nitric Oxide-Dependent Pathways as Critical Factors in the Consequences and Recovery after Brain Ischemic Hypoxia.
Wierońska JM, Cieślik P, Kalinowski L. Wierońska JM, et al. Biomolecules. 2021 Jul 26;11(8):1097. doi: 10.3390/biom11081097. Biomolecules. 2021. PMID: 34439764 Free PMC article. Review. - New progress in understanding roles of nitric oxide during hepatic ischemia-reperfusion injury.
Zhang YP, Liu XR, Yang MW, Yang SL, Hong FF. Zhang YP, et al. World J Hepatol. 2022 Mar 27;14(3):504-515. doi: 10.4254/wjh.v14.i3.504. World J Hepatol. 2022. PMID: 35582289 Free PMC article. Review.
Cited by
- Gemfibrozil Improves Microcirculatory Oxygenation of Colon and Liver without Affecting Mitochondrial Function in a Model of Abdominal Sepsis in Rats.
Kuebart A, Gross K, Maicher C, Sonnenschein M, Raupach A, Schulz J, Truse R, Hof S, Marcus C, Vollmer C, Bauer I, Picker O, Relja B, Herminghaus A. Kuebart A, et al. Int J Mol Sci. 2023 Dec 23;25(1):262. doi: 10.3390/ijms25010262. Int J Mol Sci. 2023. PMID: 38203431 Free PMC article. - Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets.
Zhang M, Liu Q, Meng H, Duan H, Liu X, Wu J, Gao F, Wang S, Tan R, Yuan J. Zhang M, et al. Signal Transduct Target Ther. 2024 Jan 8;9(1):12. doi: 10.1038/s41392-023-01688-x. Signal Transduct Target Ther. 2024. PMID: 38185705 Free PMC article. Review. - Combined Pulsed Magnetic Field and Radiofrequency Electromagnetic Field Enhances MMP-9, Collagen-4, VEGF Synthesis to Improve Wound Healing Via Hif-1α/eNOS Pathway.
Asci H, Savran M, Comlekci S, Sofu MM, Erzurumlu Y, Ozmen O, Kaynak M, Sahin ME, Taner R, Gecin M. Asci H, et al. Aesthetic Plast Surg. 2023 Dec;47(6):2841-2852. doi: 10.1007/s00266-023-03450-8. Epub 2023 Jun 27. Aesthetic Plast Surg. 2023. PMID: 37369865 - Effects of iNOS in Hepatic Warm Ischaemia and Reperfusion Models in Mice and Rats: A Systematic Review and Meta-Analysis.
Nakatake R, Schulz M, Kalvelage C, Benstoem C, Tolba RH. Nakatake R, et al. Int J Mol Sci. 2022 Oct 7;23(19):11916. doi: 10.3390/ijms231911916. Int J Mol Sci. 2022. PMID: 36233220 Free PMC article. Review. - Synergetic protective effect of remote ischemic preconditioning and prolyl 4‑hydroxylase inhibition in ischemic cardiac injury.
Yang J, Xu J, Tao L, Wang S, Xiang H, Tang Y. Yang J, et al. Mol Med Rep. 2022 Mar;25(3):80. doi: 10.3892/mmr.2022.12596. Epub 2022 Jan 14. Mol Med Rep. 2022. PMID: 35029283 Free PMC article.
References
- Oshima Y, Fujio Y, Nakanishi T, Itoh N, Yamamoto Y, Negoro S, Tanaka K, Kishimoto T, Kawase I, Azuma J. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc Res. 2005;65:428–435. doi: 10.1016/j.cardiores.2004.10.021. - DOI - PubMed
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:579–588. - PubMed
- Lønborg J, Kelbaek H, Vejlstrup N, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Treiman M, Jensen JS, Engstrøm T. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3:34–41. doi: 10.1161/CIRCINTERVENTIONS.109.905521. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous