High fat diet-induced changes in mouse muscle mitochondrial phospholipids do not impair mitochondrial respiration despite insulin resistance - PubMed (original) (raw)
High fat diet-induced changes in mouse muscle mitochondrial phospholipids do not impair mitochondrial respiration despite insulin resistance
Joris Hoeks et al. PLoS One. 2011.
Abstract
Background: Type 2 diabetes mellitus and muscle insulin resistance have been associated with reduced capacity of skeletal muscle mitochondria, possibly as a result of increased intake of dietary fat. Here, we examined the hypothesis that a prolonged high-fat diet consumption (HFD) increases the saturation of muscle mitochondrial membrane phospholipids causing impaired mitochondrial oxidative capacity and possibly insulin resistance.
Methodology: C57BL/6J mice were fed an 8-week or 20-week low fat diet (10 kcal%; LFD) or HFD (45 kcal%). Skeletal muscle mitochondria were isolated and fatty acid (FA) composition of skeletal muscle mitochondrial phospholipids was analyzed by thin-layer chromatography followed by GC. High-resolution respirometry was used to assess oxidation of pyruvate and fatty acids by mitochondria. Insulin sensitivity was estimated by HOMA-IR.
Principal findings: At 8 weeks, mono-unsaturated FA (16∶1n7, 18∶1n7 and 18∶1n9) were decreased (-4.0%, p<0.001), whereas saturated FA (16∶0) were increased (+3.2%, p<0.001) in phospholipids of HFD vs. LFD mitochondria. Interestingly, 20 weeks of HFD descreased mono-unsaturated FA while n-6 poly-unsaturated FA (18∶2n6, 20∶4n6, 22∶5n6) showed a pronounced increase (+4.0%, p<0.001). Despite increased saturation of muscle mitochondrial phospholipids after the 8-week HFD, mitochondrial oxidation of both pyruvate and fatty acids were similar between LFD and HFD mice. After 20 weeks of HFD, the increase in n-6 poly-unsaturated FA was accompanied by enhanced maximal capacity of the electron transport chain (+49%, p = 0.002) and a tendency for increased ADP-stimulated respiration, but only when fuelled by a lipid-derived substrate. Insulin sensitivity in HFD mice was reduced at both 8 and 20 weeks.
Conclusions/interpretation: Our findings do not support the concept that prolonged HF feeding leads to increased saturation of skeletal muscle mitochondrial phospholipids resulting in a decrease in mitochondrial fat oxidative capacity and (muscle) insulin resistance.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
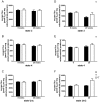
Figure 1. Respiration rates of isolated mitochondria from mouse skeletal muscle on pyruvate and palmitoyl-CoA+carnitine.
(A) ADP-stimulated (state 3) respiration on pyruvate, (B) oligomycin-insensitive (state 4) respiration on pyruvate, (C) maximally uncoupled (state UnC) respiration on pyruvate, (D) state 3 respiration on palmitoylCoA + carnitine, (E) state 4 respiration on palmitoyl-CoA + carnitine and (F) State UnC respiration on palmitoyl-CoA + carnitine. Black and white bars represent LFD mice and HFD mice, respectively. Values are means ± SE (n = 7–8). D, significant diet effect with p<0.01; T, significant time effect with p<0.05 in state 3 and state 4 and with p<0.01 in state UnC; D*T, significant diet * time effect with p<0.05; HFD, high fat diet; LFD, low fat diet; TA, tibialis anterior; UnC, uncoupled.
Similar articles
- Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance.
Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, Connor T, Watt MJ, Carpenter K, Hargreaves M, McGee SL, Hevener AL, Febbraio MA. Henstridge DC, et al. Diabetes. 2014 Jun;63(6):1881-94. doi: 10.2337/db13-0967. Epub 2014 Jan 15. Diabetes. 2014. PMID: 24430435 Free PMC article. - Remodeling of skeletal muscle mitochondrial proteome with high-fat diet involves greater changes to β-oxidation than electron transfer proteins in mice.
Dasari S, Newsom SA, Ehrlicher SE, Stierwalt HD, Robinson MM. Dasari S, et al. Am J Physiol Endocrinol Metab. 2018 Oct 1;315(4):E425-E434. doi: 10.1152/ajpendo.00051.2018. Epub 2018 May 29. Am J Physiol Endocrinol Metab. 2018. PMID: 29812987 Free PMC article. - Impact of 4 weeks of western diet and aerobic exercise training on whole-body phenotype and skeletal muscle mitochondrial respiration in male and female mice.
McGowan EM, Ehrlicher SE, Stierwalt HD, Robinson MM, Newsom SA. McGowan EM, et al. Physiol Rep. 2022 Dec;10(24):e15543. doi: 10.14814/phy2.15543. Physiol Rep. 2022. PMID: 36541261 Free PMC article. - Skeletal muscle "mitochondrial deficiency" does not mediate insulin resistance.
Holloszy JO. Holloszy JO. Am J Clin Nutr. 2009 Jan;89(1):463S-6S. doi: 10.3945/ajcn.2008.26717C. Epub 2008 Dec 3. Am J Clin Nutr. 2009. PMID: 19056574 Review. - Skeletal Muscle Nucleo-Mitochondrial Crosstalk in Obesity and Type 2 Diabetes.
Devarshi PP, McNabney SM, Henagan TM. Devarshi PP, et al. Int J Mol Sci. 2017 Apr 14;18(4):831. doi: 10.3390/ijms18040831. Int J Mol Sci. 2017. PMID: 28420087 Free PMC article. Review.
Cited by
- Saturated fatty acids negatively affect musculoskeletal tissues in vitro and in vivo.
Lin RT, Osipov B, Steffen D, Chamberlin M, Pathak SJ, Christiansen BA, Paulussen KJM, Baar K. Lin RT, et al. Matrix Biol Plus. 2024 May 31;23:100153. doi: 10.1016/j.mbplus.2024.100153. eCollection 2024 Aug. Matrix Biol Plus. 2024. PMID: 38882396 Free PMC article. - Challenging of AS160/TBC1D4 Alters Intracellular Lipid milieu in L6 Myotubes Incubated With Palmitate.
Mikłosz A, Łukaszuk B, Żendzian-Piotrowska M, Brańska-Januszewska J, Ostrowska H, Chabowski A. Mikłosz A, et al. J Cell Physiol. 2017 Sep;232(9):2373-2386. doi: 10.1002/jcp.25632. Epub 2017 Mar 31. J Cell Physiol. 2017. PMID: 27714805 Free PMC article. - Krill Oil Ameliorates Mitochondrial Dysfunctions in Rats Treated with High-Fat Diet.
Ferramosca A, Conte A, Zara V. Ferramosca A, et al. Biomed Res Int. 2015;2015:645984. doi: 10.1155/2015/645984. Epub 2015 Aug 2. Biomed Res Int. 2015. PMID: 26301251 Free PMC article. - Dietary enrichment with fish oil prevents high fat-induced metabolic dysfunction in skeletal muscle in mice.
Philp LK, Heilbronn LK, Janovska A, Wittert GA. Philp LK, et al. PLoS One. 2015 Feb 6;10(2):e0117494. doi: 10.1371/journal.pone.0117494. eCollection 2015. PLoS One. 2015. PMID: 25658742 Free PMC article. - Synergy in free radical generation is blunted by high-fat diet induced alterations in skeletal muscle mitochondrial metabolism.
Li Y, Periwal V. Li Y, et al. Biophys J. 2013 Mar 5;104(5):1127-41. doi: 10.1016/j.bpj.2013.01.025. Biophys J. 2013. PMID: 23473496 Free PMC article.
References
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Jeneson JA, Backes WH, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–120. - PubMed
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. - PubMed
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous