Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation - PubMed (original) (raw)
Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation
Xiang Zhong et al. Cell Stress Chaperones. 2012 Jul.
Abstract
Neonates with intrauterine growth retardation (IUGR) are susceptible to decreases in cellular immunity. In recent years, a growing body of evidence indicates that Hsp70 may serve as a danger signal to the innate immune system and promote receptor-mediated apoptosis. Using neonatal pigs with IUGR, we investigated immune function of pigs and expression of heat shock protein 70 (Hsp70), nuclear factor-kappa B (NF-κB), and forkhead box O 3a (FoxO3a) in the intestinal tract. Samples from the blood, duodenum, jejunum, and ileum of normal body weight (NBW) piglets and IUGR piglets were collected at day 7 after birth. Furthermore, to test whether Hsp70 is associated with regulation of NF-κB and FoxO3a, Hsp70 was silenced using small RNA interference (siRNA) in IEC-6 cells. Body and intestinal weights were lower in IUGR piglets than in NBW piglets (p < 0.05). Proliferation of peripheral blood lymphocytes was decreased (p < 0.05) in IUGR piglets. Cytokine concentrations (IFN-γ, IL-4, IL-10, IL-1, and IL-8) were lower in serum of IUGR piglets. The levels of IFN-γ and IL-10 were decreased (p < 0.05) in the ileum of IUGR piglets, but IL-4 was increased (p < 0.05). The expressions of Hsp70 and FoxO3a were increased, and NF-κB activity was downregulated in IUGR piglets (p < 0.05). Furthermore, siRNA-mediated Hsp70 downregulation increased NF-κB activity, inhibited expression of FoxO3a, and decreased cell apoptosis. In contrast, overexpression of Hsp70 inhibited NF-κB activation. In conclusion, IUGR impairs immune functions in neonatal pigs. An inefficient immunity in IUGR piglets is associated with overexpression of Hsp70, which impairs NF-κB signaling and upregulates FoxO3a expression.
Figures
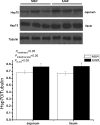
Fig. 1
Expression of Hsp70 in the jejunum and ileum of NBW piglets and IUGR piglets. Values are means±SEM, n = 5 per treatment. Mean differences between values for IUGR piglets and NBW piglets are indicated by *P < 0.05. P_treatment_P value for treatment, P_intestinal site_P value for intestinal site, P_tr×int_P value for interaction between treatment and intestinal site
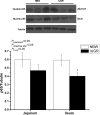
Fig. 2
Expression of the NF-κB p65 subunits in the jejunum and ileum of NBW piglets and IUGR piglets. Values are means±SEM, n = 5 per treatment. Mean differences between values for IUGR piglets and NBW piglets are indicated by *P < 0.05. P_treatment_P value for treatment, P_intestinal site_P value for intestinal site, P_tr×int_P value for interaction between treatment and intestinal site
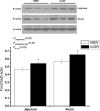
Fig. 3
Expression of FoxO3a in the jejunum and ileum of NBW piglets and IUGR piglets. Values are means±SEM, n = 5 per treatment. Mean differences between values for IUGR piglets and NBW piglets are indicated by *P < 0.05. P_treatment_P value for treatment, P_intestinal site_P value for intestinal site, P_tr×int_P value for interaction between treatment and intestinal site
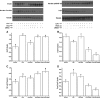
Fig. 4
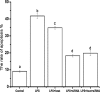
Activity of IKK (a), concentrations of IκB (b), activity of NF-κB (c), and expression of FoxO3a (d) induced by LPS, heat shock, and siRNA-mediated Hsp70 gene silencing in IEC-6 cells. Values are means±SEM, n = 6 per treatment. Means without a common letter differ (P < 0.05)
Fig. 5
Apoptosis induced by LPS, heat shock, and siRNA-mediated Hsp70 gene silencing in IEC-6 cells. Values are means±SEM, n = 6 per treatment. Means without a common letter differ (P < 0.05)
Similar articles
- Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets.
Zhong X, Wang T, Zhang X, Li W. Zhong X, et al. Cell Stress Chaperones. 2010 May;15(3):335-42. doi: 10.1007/s12192-009-0148-3. Epub 2009 Oct 15. Cell Stress Chaperones. 2010. PMID: 19830596 Free PMC article. - Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs.
Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, He S, Zhang K, Che L. Han F, et al. Br J Nutr. 2013 Nov;110(10):1819-27. doi: 10.1017/S0007114513001232. Epub 2013 Apr 19. Br J Nutr. 2013. PMID: 23596997 - Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction.
Hu L, Liu Y, Yan C, Peng X, Xu Q, Xuan Y, Han F, Tian G, Fang Z, Lin Y, Xu S, Zhang K, Chen D, Wu D, Che L. Hu L, et al. Br J Nutr. 2015 Jul 14;114(1):53-62. doi: 10.1017/S0007114515001579. Epub 2015 Jun 10. Br J Nutr. 2015. PMID: 26059215 - Maternal imprinting of the neonatal microbiota colonization in intrauterine growth restricted piglets: a review.
Jiang L, Feng C, Tao S, Li N, Zuo B, Han D, Wang J. Jiang L, et al. J Anim Sci Biotechnol. 2019 Nov 11;10:88. doi: 10.1186/s40104-019-0397-7. eCollection 2019. J Anim Sci Biotechnol. 2019. PMID: 31737268 Free PMC article. Review. - Hsp70 and cardiac surgery: molecular chaperone and inflammatory regulator with compartmentalized effects.
de Jong PR, Schadenberg AW, Jansen NJ, Prakken BJ. de Jong PR, et al. Cell Stress Chaperones. 2009 Mar;14(2):117-31. doi: 10.1007/s12192-008-0066-9. Epub 2008 Jul 31. Cell Stress Chaperones. 2009. PMID: 18668350 Free PMC article. Review.
Cited by
- The effect of intrauterine growth restriction on the developing pancreatic immune system.
Golden TN, Garifallou JP, Conine CC, Simmons RA. Golden TN, et al. bioRxiv [Preprint]. 2024 Sep 23:2024.09.19.613902. doi: 10.1101/2024.09.19.613902. bioRxiv. 2024. PMID: 39386426 Free PMC article. Preprint. - Developmental origins of metabolic disorders: The need for biomarker candidates and therapeutic targets from adequate preclinical models.
Gonzalez-Bulnes A, Astiz S, Vazquez-Gomez M, Garcia-Contreras C. Gonzalez-Bulnes A, et al. EuPA Open Proteom. 2016 Jan 7;10:50-55. doi: 10.1016/j.euprot.2016.01.001. eCollection 2016 Mar. EuPA Open Proteom. 2016. PMID: 29900100 Free PMC article. - Gut epithelial inducible heat-shock proteins and their modulation by diet and the microbiota.
Arnal ME, Lallès JP. Arnal ME, et al. Nutr Rev. 2016 Mar;74(3):181-97. doi: 10.1093/nutrit/nuv104. Epub 2016 Feb 16. Nutr Rev. 2016. PMID: 26883882 Free PMC article. Review. - The role of interleukin-1 in perinatal inflammation and its impact on transitional circulation.
Owen JC, Garrick SP, Peterson BM, Berger PJ, Nold MF, Sehgal A, Nold-Petry CA. Owen JC, et al. Front Pediatr. 2023 Mar 13;11:1130013. doi: 10.3389/fped.2023.1130013. eCollection 2023. Front Pediatr. 2023. PMID: 36994431 Free PMC article. Review. - Intrauterine Growth Restriction Impairs Small Intestinal Mucosal Immunity in Neonatal Piglets.
Dong L, Zhong X, Ahmad H, Li W, Wang Y, Zhang L, Wang T. Dong L, et al. J Histochem Cytochem. 2014 Jul;62(7):510-8. doi: 10.1369/0022155414532655. Epub 2014 Apr 7. J Histochem Cytochem. 2014. PMID: 24710659 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials