Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer - PubMed (original) (raw)
Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer
Farbod Shojaei et al. J Exp Clin Cancer Res. 2012.
Abstract
Osteopontin (OPN), also known as SPP1 (secreted phosphoprotein), is an integrin binding glyco-phosphoprotein produced by a variety of tissues. In cancer patients expression of OPN has been associated with poor prognosis in several tumor types including breast, lung, and colorectal cancers. Despite wide expression in tumor cells and stroma, there is limited evidence supporting role of OPN in tumor progression and metastasis. Using phage display technology we identified a high affinity anti-OPN monoclonal antibody (hereafter AOM1). The binding site for AOM1 was identified as SVVYGLRSKS sequence which is immediately adjacent to the RGD motif and also spans the thrombin cleavage site of the human OPN. AOM1 efficiently inhibited OPNa binding to recombinant integrin αvβ3 with an IC50 of 65 nM. Due to its unique binding site, AOM1 is capable of inhibiting OPN cleavage by thrombin which has been shown to produce an OPN fragment that is biologically more active than the full length OPN. Screening of human cell lines identified tumor cells with increased expression of OPN receptors (αvβ3 and CD44v6) such as mesothelioma, hepatocellular carcinoma, breast, and non-small cell lung adenocarcinoma (NSCLC). CD44v6 and αvβ3 were also found to be highly enriched in the monocyte, but not lymphocyte, subset of human peripheral blood mononuclear cells (hPBMCs). In vitro, OPNa induced migration of both tumor and hPBMCs in a transwell migration assay. AOM1 significantly blocked cell migration further validating its specificity for the ligand. OPN was found to be enriched in mouse plasma in a number of pre-clinical tumor model of non-small cell lung cancers. To assess the role of OPN in tumor growth and metastasis and to evaluate a potential therapeutic indication for AOM1, we employed a Kras(G12D-LSL)p53(fl/fl) subcutaneously implanted in vivo model of NSCLC which possesses a high capacity to metastasize into the lung. Our data indicated that treatment of tumor bearing mice with AOM1 as a single agent or in combination with Carboplatin significantly inhibited growth of large metastatic tumors in the lung further supporting a role for OPN in tumor metastasis and progression.
Figures
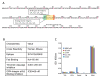
Figure 1
Development of anti-OPN antibody. A Amino acid sequence of OPNa (full length OPN). Truncated isoforms OPNb and OPNc are highlighted with blue and yellow, respectively. Binding sites for integrins are highlighted with green (RGD binding integrins) and orange (LDV binding integrins). Thrombin cleavage site is marked by a red arrow. B Characterization of AOM1 including its cross-reactivity, binding epitope, dissociation constant (KD) for the Fab and its ability to inhibit binding of recombinant OPNa to immobilized integrin αvβ3 have been determined. C Selectivity of AOM1 for human OPN over other RGD-motif containing proteins was assessed by ELISA as detailed in Materials and Methods. RGD containing proteins were immobilized on an immunosorbent plate and binding of AOM1 assessed at 0.1, 1, 10 and 1000 nM concentrations. With the exception of 1000 nM AOM1 vs. ColA1, there was no binding observed at any concentration of AOM1 up to 1000 nM versus thrombospondin, vitronectin, ColA1 and fibronectin whilst saturated binding was observed vs. OPN at antibody concentrations as low as 0.1 nM AOM1. Each bar represents mean OD450 nm value of triplicate measurements with standard error bars.
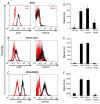
Figure 2
OPN act as a chemotactic factor in human cells lines expressing OPN receptors. A-C Using flowcytometry expression of OPN receptor, mainly CD44v6 and αvβ3 was assessed in series of human cell lines. Three cell types found to have greater expression of one or both receptors. These lines include JHH4 hepatocellular (A) carcinoma, MSTO211H mesothelioma (B) and MDA-MB435 melanoma cells (C). D-F Migration assay provided functional relevance for expression of OPN receptors in the above cell lines. Using transwell, each cell line was added to the top chamber and its migration towards OPN was evaluated.
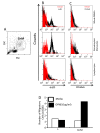
Figure 3
CD44v6 and αvβ3 are highly expressed in granulocyte and monocyte but not lymphocyte subpopulation of hPBMCs. A Representative side scatter vs. forward scatter plot of hPBMCs representing populations of lymphocytes (L), granulocytes (G) and monocytes (M). B&C Expression of OPN receptors (αvβ3 (B) and CD44v6 (D)) was measured in hPBMCs and was evaluated in L vs. GM subsets. D Transwell migration assay in L vs. GM subset indicated that only the latter is capable of migrating toward OPN thus providing a functional relevance of expression of receptors.
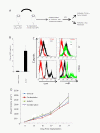
Figure 4
Characterizing OPN and its receptors in mouse NSCLCs. A Development of KPT model. KrasG12D-LSLp53fl/fl (KP) mice were inhaled with Adeno-CMV-Cre at approximately 8 weeks after birth. Lung tumors were inspected at approximately 18 weeks post-inhalation. Pieces of lung tumors were taken from transgenic mice and were implanted subcutaneously (without any in vitro manipulation) into Scid/beige mice using trocar to generate KPT (KrasG12D-LSLp53fl/fl trocar) model as described in the Materials and Methods. B Tumor implantation results in increased levels of OPN in the plasma in tumor bearing mice. C Using flowcytometry, expression of CD44v6 and αvβ3 was evaluated in KP cells and mPBMCs. Cells were stained with the antibodies as described in materials and methods and data analysis showed greater expression of αvβ3 than CD44 in both KP and mPBMCs. D KPT mice were randomized and received treatments (Vehicle, AOM1, Carboplatin and combination) at 8 days post-implantation. Tumors volume were measured twice/week and study was terminated at 27 days after implantation.
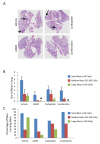
Figure 5
AOM1 inhibits growth of large tumors in the lung in a NSCLC tumor. A Scid/beige mice were sc implanted with pieces of tumors isolated from lung lesions from KrasG12D-LSLp53fl/fl mice. Implanted mice were randomized at 8 days post-implantation and were treated with vehicle, AOM1, carboplatin and combination of both compounds. Tumor volume was measured using caliper twice per week. At terminal analysis whole lung from each mouse was fixed in formalin and was stained in H&E. Representative images from each treatment are shown. In pathology analysis lung lesions were classified into small (less than 10 cells) medium (10-200) and large (more than 200 cells) size and were quantified in each treatment. B Quantifications of lesions in each treatment. Bar graph represents mean number of lesions ± SEM. C Frequency of mice carrying each lesion in each treatment also indicated that AOM1 as single agent or in combination with Carboplatin significantly inhibits percentage of mice carrying large tumors in the lung.
Similar articles
- Thrombin cleavage of osteopontin disrupts a pro-chemotactic sequence for dendritic cells, which is compensated by the release of its pro-chemotactic C-terminal fragment.
Shao Z, Morser J, Leung LLK. Shao Z, et al. J Biol Chem. 2014 Sep 26;289(39):27146-27158. doi: 10.1074/jbc.M114.572172. Epub 2014 Aug 11. J Biol Chem. 2014. PMID: 25112870 Free PMC article. - Abrogation of the interaction between osteopontin and alphavbeta3 integrin reduces tumor growth of human lung cancer cells in mice.
Cui R, Takahashi F, Ohashi R, Gu T, Yoshioka M, Nishio K, Ohe Y, Tominaga S, Takagi Y, Sasaki S, Fukuchi Y, Takahashi K. Cui R, et al. Lung Cancer. 2007 Sep;57(3):302-10. doi: 10.1016/j.lungcan.2007.03.019. Epub 2007 May 4. Lung Cancer. 2007. PMID: 17482311 - Deletion of the thrombin cleavage domain of osteopontin mediates breast cancer cell adhesion, proteolytic activity, tumorgenicity, and metastasis.
Beausoleil MS, Schulze EB, Goodale D, Postenka CO, Allan AL. Beausoleil MS, et al. BMC Cancer. 2011 Jan 19;11:25. doi: 10.1186/1471-2407-11-25. BMC Cancer. 2011. PMID: 21247495 Free PMC article. - Thrombin Cleavage of Osteopontin and the Host Anti-Tumor Immune Response.
Leung LL, Myles T, Morser J. Leung LL, et al. Cancers (Basel). 2023 Jul 3;15(13):3480. doi: 10.3390/cancers15133480. Cancers (Basel). 2023. PMID: 37444590 Free PMC article. Review. - Prognostic and clinicopathological value of osteopontin expression in non-small cell lung cancer: a meta-analysis and systematic review.
Song Y, Li H, Jiang Q, Wu L. Song Y, et al. Biomarkers. 2024 Mar;29(2):105-113. doi: 10.1080/1354750X.2024.2319702. Epub 2024 Feb 29. Biomarkers. 2024. PMID: 38376506 Review.
Cited by
- Peptide-based vaccination against OPN integrin binding sites does not improve cardio-metabolic disease in mice.
Grün NG, Strohmeier K, Moreno-Viedma V, Le Bras M, Landlinger C, Zeyda K, Wanko B, Leitner L, Staffler G, Zeyda M, Stulnig TM. Grün NG, et al. Immunol Lett. 2016 Nov;179:85-94. doi: 10.1016/j.imlet.2016.09.006. Epub 2016 Sep 14. Immunol Lett. 2016. PMID: 27639826 Free PMC article. - Comparative effectiveness of radiofrequency ablation with or without transarterial chemoembolization for hepatocellular carcinoma.
Kong QF, Jiao JB, Chen QQ, Li L, Wang DG, Lv B. Kong QF, et al. Tumour Biol. 2014 Mar;35(3):2655-9. doi: 10.1007/s13277-013-1349-z. Epub 2013 Nov 7. Tumour Biol. 2014. PMID: 24197985 - Osteopontin b and c Splice isoforms in Leukemias and Solid Tumors: Angiogenesis Alongside Chemoresistance.
Mirzaei A, Mohammadi S, Ghaffari SH, Yaghmaie M, Vaezi M, Alimoghaddam K, Ghavamzadeh A. Mirzaei A, et al. Asian Pac J Cancer Prev. 2018 Mar 27;19(3):615-623. doi: 10.22034/APJCP.2018.19.3.615. Asian Pac J Cancer Prev. 2018. PMID: 29580029 Free PMC article. Review. - Role of osteopontin in cancer development and treatment.
Yan Z, Hu X, Tang B, Deng F. Yan Z, et al. Heliyon. 2023 Oct 14;9(10):e21055. doi: 10.1016/j.heliyon.2023.e21055. eCollection 2023 Oct. Heliyon. 2023. PMID: 37867833 Free PMC article. Review. - Assessing the Feasibility of Neutralizing Osteopontin with Various Therapeutic Antibody Modalities.
Farrokhi V, Chabot JR, Neubert H, Yang Z. Farrokhi V, et al. Sci Rep. 2018 May 17;8(1):7781. doi: 10.1038/s41598-018-26187-w. Sci Rep. 2018. PMID: 29773891 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous