Thailandepsins are new small molecule class I HDAC inhibitors with potent cytotoxic activity in ovarian cancer cells: a preclinical study of epigenetic ovarian cancer therapy - PubMed (original) (raw)
Thailandepsins are new small molecule class I HDAC inhibitors with potent cytotoxic activity in ovarian cancer cells: a preclinical study of epigenetic ovarian cancer therapy
Andrew J Wilson et al. J Ovarian Res. 2012.
Abstract
Background: New treatment strategies are emerging to target DNA damage response pathways in ovarian cancer. Our group has previously shown that the class I biased HDAC inhibitor romidepsin (FK228) induces DNA damage response and has potent cytotoxic effects in ovarian cancer cells. Here, we investigated newly discovered HDAC inhibitors, thailandepsin A (TDP-A) and thailandepsin B (TDP-B), to determine the effects on cell viability, apoptosis and DNA damage response in ovarian cancer cells.
Methods: FK228, TDP-A and TDP-B were tested in five ovarian cancer cell lines. Cellular viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays. Immunofluorescence assays were used to assess activated caspase 3. Western blots were performed to detect protein expression of PARP cleavage, pH2AX, P-glycoprotein and tubulin acetylation.
Results: Treatment with TDPs decreased cell viability at nanonomolar concentrations in four of the five ovarian cancer cell lines studied. Similar to FK228, both TDP compounds exerted minimal effects on NCI/ADR-RES ovarian cancer cells. Across the four cell lines sensitive to the TDPs, TDP-B consistently had a greater inhibitory effect than TDP-A on cell viability. TDP-B also had relatively greater effects on promoting cell apoptosis and induction of pH2AX (a mark of DNA damage response), than TDP-A. These antitumor effects of TDP-B were of similar magnitude to those induced by an equal concentration of FK228. Similar to FK228, the nanomolar concentrations of the TDPs had little effect on tubulin acetylation (a mark of class II HDAC6 inhibition).
Conclusions: The new small molecule HDAC inhibitors TDP-A and TDP-B are FK228 analogues that suppress cell viability and induce apoptosis at nanomolar drug concentrations. TDP-B showed the most similarity to the biological activity of FK228 with greater cytotoxic effects than TDP-A in vitro. Our results indicate that FK228-like small molecule class I HDAC-biased HDAC inhibitors have therapeutic potential for ovarian cancer.
Figures
Figure 1
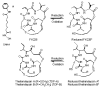
Chemical structure of HDAC inhibitors. Thailandepsin A (TDP-A), thailandepsin B (TDP-B) and FK228 are depsipeptides characterized by a bicyclic structure containing a signature disulfide bond; the prodrugs can be activated by cellular reduction, indicated by a star sign (*). SAHA is a hydroxamic acid marked by a pound sign (#)
Figure 2
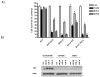
TDP-A and TDP-B inhibit ovarian cancer cell survival at nanomolar concentrations. A) Representative graphs of NCI/ADR-RES, OVCAR-8, SKOV-3, BRCA1 wild type and null ovarian cancer cells treated with FK228, TDP-A and TDP-B at a fixed concentration of 10 nM. MTT assays were performed to assess cell proliferation after 72 h of treatment. Each treatment was replicated 6 times. Values are mean + SE for 3 independent experiments. B) Representative Western blot for P-glycoprotein expression in NCI/ADR-RES, OVCAR-8 and SKOV-3 ovarian cancer cells after 24 h of treatment with 10nM FK228, 10nM TDP-A and 10nM TDP-B. 0.1% DMSO was the vehicle control. β-actin was used as a loading control.
Figure 3
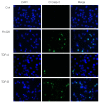
TDP-B activates cleaved caspase-3 by immunofluorescence. Representative immunofluorescence staining for cleaved caspase 3 (green) in SKOV-3 ovarian cancer cells treated with 10 nM FK228, TDP-A or TDP-B after 24 h of exposure. The nuclei are stained with DAPI (blue)
Figure 4
Representative Western blots show TDP-A and TDP-B upregulate cleaved PARP and pH2AX. Western blot analysis of A) cleaved PARP and B) pH2AX after 24 h of treatment with 10 nM FK228, TDP-A and TDP-B in NCI/ADR-RES, OVCAR-8 and SKOV-3 ovarian cancer cells. The loading controls in A) and B) were β-actin and histone H3, respectively.
Figure 5
TDPs are class I biased HDAC inhibitors and do not upregulate acetylated tubulin. A) HDAC inhibition of HDACs 1, 2, 3 and 6 measured by HDAC enzymatic assays, in which the compounds are reduced in test tubes [12]. B) Western blot analysis of acetylated tubulin in NCI/ADR-RES, OVCAR-8 and SKOV-3 ovarian cancer cells. Tubulin was the loading control.
Similar articles
- The effects of the histone deacetylase inhibitor romidepsin (FK228) are enhanced by aspirin (ASA) in COX-1 positive ovarian cancer cells through augmentation of p21.
Son DS, Wilson AJ, Parl AK, Khabele D. Son DS, et al. Cancer Biol Ther. 2010 Jun 1;9(11):928-35. doi: 10.4161/cbt.9.11.11873. Epub 2010 Jun 25. Cancer Biol Ther. 2010. PMID: 20404564 Free PMC article. - Thailandepsins: bacterial products with potent histone deacetylase inhibitory activities and broad-spectrum antiproliferative activities.
Wang C, Henkes LM, Doughty LB, He M, Wang D, Meyer-Almes FJ, Cheng YQ. Wang C, et al. J Nat Prod. 2011 Oct 28;74(10):2031-8. doi: 10.1021/np200324x. Epub 2011 Jul 27. J Nat Prod. 2011. PMID: 21793558 Free PMC article. - Romidepsin (FK228) combined with cisplatin stimulates DNA damage-induced cell death in ovarian cancer.
Wilson AJ, Lalani AS, Wass E, Saskowski J, Khabele D. Wilson AJ, et al. Gynecol Oncol. 2012 Dec;127(3):579-86. doi: 10.1016/j.ygyno.2012.09.016. Epub 2012 Sep 23. Gynecol Oncol. 2012. PMID: 23010348 Free PMC article. - Romidepsin (FK228), A Histone Deacetylase Inhibitor and its Analogues in Cancer Chemotherapy.
Pojani E, Barlocco D. Pojani E, et al. Curr Med Chem. 2021;28(7):1290-1303. doi: 10.2174/0929867327666200203113926. Curr Med Chem. 2021. PMID: 32013816 Review. - Histone deacetylase inhibitors from microorganisms: the Astellas experience.
Masuoka Y, Shindoh N, Inamura N. Masuoka Y, et al. Prog Drug Res. 2008;66:335, 337-59. doi: 10.1007/978-3-7643-8595-8_7. Prog Drug Res. 2008. PMID: 18416310 Review.
Cited by
- HDAC6 and ovarian cancer.
Haakenson J, Zhang X. Haakenson J, et al. Int J Mol Sci. 2013 May 2;14(5):9514-35. doi: 10.3390/ijms14059514. Int J Mol Sci. 2013. PMID: 23644884 Free PMC article. Review. - Disulfide cross-linked micelles of novel HDAC inhibitor thailandepsin A for the treatment of breast cancer.
Xiao K, Li YP, Wang C, Ahmad S, Vu M, Kuma K, Cheng YQ, Lam KS. Xiao K, et al. Biomaterials. 2015 Oct;67:183-93. doi: 10.1016/j.biomaterials.2015.07.033. Epub 2015 Jul 17. Biomaterials. 2015. PMID: 26218744 Free PMC article. - Histone deacetylases and mechanisms of regulation of gene expression.
Chen HP, Zhao YT, Zhao TC. Chen HP, et al. Crit Rev Oncog. 2015;20(1-2):35-47. doi: 10.1615/critrevoncog.2015012997. Crit Rev Oncog. 2015. PMID: 25746103 Free PMC article. Review. - Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid.
Yuille S, Reichardt N, Panda S, Dunbar H, Mulder IE. Yuille S, et al. PLoS One. 2018 Jul 27;13(7):e0201073. doi: 10.1371/journal.pone.0201073. eCollection 2018. PLoS One. 2018. PMID: 30052654 Free PMC article. - The novel histone deacetylase inhibitor thailandepsin A inhibits anaplastic thyroid cancer growth.
Weinlander E, Somnay Y, Harrison AD, Wang C, Cheng YQ, Jaskula-Sztul R, Yu XM, Chen H. Weinlander E, et al. J Surg Res. 2014 Jul;190(1):191-7. doi: 10.1016/j.jss.2014.02.042. Epub 2014 Feb 28. J Surg Res. 2014. PMID: 24679699 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials