Beta-catenin inhibits melanocyte migration but induces melanoma metastasis - PubMed (original) (raw)
Beta-catenin inhibits melanocyte migration but induces melanoma metastasis
S J Gallagher et al. Oncogene. 2013.
Abstract
The canonical Wnt signalling pathway induces the β-catenin/lymphoid enhancer factor transcription factors. It is activated in various cancers, most characteristically carcinomas, in which it promotes metastatic spread by increasing migration and/or invasion. The Wnt/β-catenin signalling pathway is frequently activated in melanoma, but the presence of β-catenin in the nucleus does not seem to be a sign of aggressiveness in these tumours. We found that, unlike its positive role in stimulating migration and invasion of carcinoma cells, β-catenin signalling decreased the migration of melanocytes and melanoma cell lines. In vivo, β-catenin signalling in melanoblasts reduced the migration of these cells, causing a white belly-spot phenotype. The inhibition by β-catenin of migration was dependent on MITF-M, a key transcription factor of the melanocyte lineage, and CSK, an Src-inhibitor. Despite reducing migration, β-catenin signalling promoted lung metastasis in the NRAS-driven melanoma murine model. Thus, β-catenin may have conflicting roles in the metastatic spread of melanoma, repressing migration while promoting metastasis. These results highlight that metastasis formation requires a series of successful cellular processes, any one of which may not be optimally efficient.
Conflict of interest statement
Conflict of interest
We do not have any conflict of interest to declare
Figures
Figure 1. Wnt/β-catenin signalling pathway and migration of melanocytes
(A) The canonical β-catenin activity of a panel of human melanoma cell lines was measured in the TOP flash assay, according to (31), with each result standardised so that the FOP flash was equal to one. TOP flash activity was compared with cell migration speed in a scratch wound assay. Standard deviations are included. We calculated the Spearman correlation coefficient of −0.82 showing an inverse tendency between TOP flash activity and cell migration. The results were considered as significant since r < −0.5. All cell lines were transfected with TOP flash vector and with the pPGKB-geobpA construct as a loading control (32). (B) Melan-a murine melanocytes were treated with 10 mM NaCl (control) or LiCl and their migration was measured in a scratch wound assay. Representative pictures of the size of the scratch wound 0 and 14 h after wounding are shown. Black lines indicate the starting positions of the cells. Inset: magnification of the outlined area. Scale bars: 50 µm. (C) The percentage of closure for each wound was evaluated from microscopy images at 30-minute intervals. Experiments were performed three times, with six replicates in each experiment. Error bars: 95% confidence interval of the mean. At 14 hours, _p_-value <10−4.
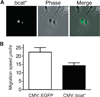
Figure 2. Nuclear β-catenin represses the migration of melan-a murine melanocytes
(A) Live-cell microscopy of melan-a murine melanocytes transfected with a vector expressing for bcat*, an EGFP-tagged β-catenin molecule targeted to the nucleus by an NLS and resistant to CK1/GSK-3β phosphorylation due to four mutations of its N terminal serine/threonine residues (at positions 33, 37, 41, and 45). (B) bcat* transfection reduced the random cell migration of melan-a cells plated at low density. Error bars: 95% confidence interval of the mean, _p_-value =1×10−3.
Figure 3. Activated β-catenin expression in the melanocyte lineage in vivo impairs the dorsolateral migration of melanoblasts and results in a white belly phenotype
(A) Map of the Tyr:: β-cat-mut-nls-egfp (bcat*) transgene. (B, C) White belly patch in a 5-month old bcat* mouse and a WT mouse. (D, E) X-gal staining revealing the absence of ventral melanoblasts at embryonic stage E15.5 in bcat* mice. The embryos contain the Dct-LacZ transgene restricting lacZ expression to the melanoblasts. (F–K) Whole-mount photographs of X-gal-stained melanoblasts in the flank region of WT-LacZ and bcat*-LacZ mouse embryos at stages E10.5, E11.5 and E14.5. Arrows indicate the direction of melanoblast migration. (L) Embryos between embryonic stages E12.5 and E15.5 were transversely sectioned through the trunk region, between somites 16 and 23, and the distance migrated by the most advanced melanoblast from the dorsal tip was measured (M) and plotted against time (N). Error bars: standard deviation. Linear regression, difference in slope _p_-value: 0.037
Figure 4. Immortalised melanocyte cell lines established from bcat* neonates contain higher levels of MITF-M and mDia1 and migrate more slowly than WT melanocytes
(A) Western blot analysis of β-catenin, MITF-M and mDia1 proteins in WT and bcat* melanocyte cell lines. st and lg indicate that the exposure time are short and long, respectively. The arrow indicates bcat* protein, which is larger than the endogenous β-catenin (darker bands lower down the blot) due to the NLS and EGFP tags. •-tubulin: loading control. (B) WT and bcat* melanocyte cell lines were transfected with TOP flash vector and with the pPGKB-geobpA construct as a loading control (32). _p_-value = 0.002. (C) The mean migration velocity of the WT and bcat* cell lines was calculated from cells grown at low-density and tracked by live imaging, at 4-minute intervals, over 12 hours. Experiments were performed 3 times, with at least 30 cells tracked per experiment. Error bars: 95% confidence interval of the mean. _p_-value <10−4.
Figure 5. bcat* has a partial requirement for MITF-M to inhibit migration
(A) The random migration speed of Mull human melanoma cells transfected with MITF-M or control siRNA, and a vector expressing the EGFP-tagged bcat* or EGFP, was measured by manually tracking transfected cells plated at low density. Asterisks above lines indicate a significant difference between treatments (Tukey test). Experiments were performed three times, with at least 80 cells tracked per treatment. (B) The abundance of MITF-M and bcat* proteins in the cells tracked in panel A was assessed by western blotting. bcat* and GFP was detected with an antibody against GFP. We did not expect the effects of bcat* expression on MITF-M levels to be detectable on western blots due to the low percentage of transfected cells expressing the bcat* vector. (C) The size of the white belly spot in WT (+), MITFvga9/+ (=mi), bcat* (=bcat), and MITFvga9/+; bcat* (=mi;bcat) mice was measured and is expressed as a percentage of the belly area between the four limbs. Error bars: standard deviation.
Figure 6. Reduction of CSK in human melanoma cell lines induces wound closure
(A) Dauv-1 human melanoma cells were transfected with 100 nM siRNA scramble (si scr) or 100 nM siRNA directed against CSK (si CSK) and their migration was measured in a scratch wound assay. Representative pictures of the size of the scratch wound 0 and 15 h after wounding are shown. Red lines indicate the front of migration of the cells. (B) The percentage of closure for each wound was evaluated from microscopy images at 30-minute intervals. Experiments were performed three times, with six replicates in each experiment. Error bars: 95% confidence interval of the mean. _p_-values were estimated at different times: p-value at 3 hours = 10−4, p-value at 6 hours = 2 10−3 and p-value at 15 hours = 10−2. (C) The abundance of CSK, SRC and its phosphorylated forms in the cells tracked in panel A was assessed by western blotting. The phosphorylation of tyrosine 416 of Src (SRCYP416a) upregulates its kinase activity whereas the phosphorylation of tyrosine 527 of Src (SRCYP527i) renders the enzyme less active. Actin was as loading control.
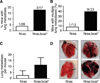
Figure 7. bcat* increases the metastatic potential of murine melanoma
(A) The lungs of the Nras and Nras;bcat* mice that developed primary melanoma were investigated for distant metastases. (_p_-value = 0.28, Fisher’s exact test). (B) The number of nude mice with multiple lung tumours was scored after the injection of 2.5 × 105 cells from Nras or Nras;bcat* tumours into the tail vein. χ2 = 0.043. (C) Mean number of lung metastases per nude mouse presenting with lung tumours after the injection of Nras or Nras;bcat* melanoma cells into the tail vein (_p_-value = 0.024). (D) Lungs from nude mice, following the injection of cells from Nras or Nras;bcat* tumours into the tail vein. Arrows show melanoma tumours. The lungs shown were removed from mice about 100 days after the injection of Nras or Nras;bcat* cells. OCT was injected into the lungs to facilitate the visualisation and preservation of lung tissue.
Similar articles
- The WNT/Beta-catenin pathway in melanoma.
Larue L, Delmas V. Larue L, et al. Front Biosci. 2006 Jan 1;11:733-42. doi: 10.2741/1831. Front Biosci. 2006. PMID: 16146765 Review. - Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: implication in cell differentiation.
Bellei B, Pitisci A, Catricalà C, Larue L, Picardo M. Bellei B, et al. Pigment Cell Melanoma Res. 2011 Apr;24(2):309-25. doi: 10.1111/j.1755-148X.2010.00800.x. Epub 2011 Jan 11. Pigment Cell Melanoma Res. 2011. PMID: 21040502 - Exploiting Honokiol-induced ER stress CHOP activation inhibits the growth and metastasis of melanoma by suppressing the MITF and β-catenin pathways.
Chiu CS, Tsai CH, Hsieh MS, Tsai SC, Jan YJ, Lin WY, Lai DW, Wu SM, Hsing HY, Arbiser JL, Sheu ML. Chiu CS, et al. Cancer Lett. 2019 Feb 1;442:113-125. doi: 10.1016/j.canlet.2018.10.026. Epub 2018 Nov 1. Cancer Lett. 2019. PMID: 30391358 - WLS inhibits melanoma cell proliferation through the β-catenin signalling pathway and induces spontaneous metastasis.
Yang PT, Anastas JN, Toroni RA, Shinohara MM, Goodson JM, Bosserhoff AK, Chien AJ, Moon RT. Yang PT, et al. EMBO Mol Med. 2012 Dec;4(12):1294-307. doi: 10.1002/emmm.201201486. Epub 2012 Nov 6. EMBO Mol Med. 2012. PMID: 23129487 Free PMC article. - A Wnt-er migration: the confusing role of β-catenin in melanoma metastasis.
Webster MR, Weeraratna AT. Webster MR, et al. Sci Signal. 2013 Mar 26;6(268):pe11. doi: 10.1126/scisignal.2004114. Sci Signal. 2013. PMID: 23532332 Review.
Cited by
- The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment.
Marrapodi R, Bellei B. Marrapodi R, et al. Cancers (Basel). 2024 Feb 23;16(5):913. doi: 10.3390/cancers16050913. Cancers (Basel). 2024. PMID: 38473275 Free PMC article. Review. - Phenotype Switching and the Melanoma Microenvironment; Impact on Immunotherapy and Drug Resistance.
Hossain SM, Eccles MR. Hossain SM, et al. Int J Mol Sci. 2023 Jan 13;24(2):1601. doi: 10.3390/ijms24021601. Int J Mol Sci. 2023. PMID: 36675114 Free PMC article. Review. - Nuclear Expression of β-Catenin Is Associated with Improved Outcomes in Endometrial Cancer.
Masciullo V, Susini T, Corrado G, Stepanova M, Baroni A, Renda I, Castiglione F, Minimo C, Bellacosa A, Chiofalo B, Vizza E, Scambia G. Masciullo V, et al. Diagnostics (Basel). 2022 Oct 3;12(10):2401. doi: 10.3390/diagnostics12102401. Diagnostics (Basel). 2022. PMID: 36292090 Free PMC article. - Stepwise-edited, human melanoma models reveal mutations' effect on tumor and microenvironment.
Hodis E, Torlai Triglia E, Kwon JYH, Biancalani T, Zakka LR, Parkar S, Hütter JC, Buffoni L, Delorey TM, Phillips D, Dionne D, Nguyen LT, Schapiro D, Maliga Z, Jacobson CA, Hendel A, Rozenblatt-Rosen O, Mihm MC Jr, Garraway LA, Regev A. Hodis E, et al. Science. 2022 Apr 29;376(6592):eabi8175. doi: 10.1126/science.abi8175. Epub 2022 Apr 29. Science. 2022. PMID: 35482859 Free PMC article. - NSAIDs and Cancer Resolution: New Paradigms beyond Cyclooxygenase.
Kolawole OR, Kashfi K. Kolawole OR, et al. Int J Mol Sci. 2022 Jan 27;23(3):1432. doi: 10.3390/ijms23031432. Int J Mol Sci. 2022. PMID: 35163356 Free PMC article. Review.
References
- Lugli A, Zlobec I, Minoo P, Baker K, Tornillo L, Terracciano L, et al. Prognostic significance of the wnt signalling pathway molecules APCbeta-catenin and E-cadherin in colorectal cancer: a tissue microarray-based analysis. Histopathology. [Research Support, Non-U.S. Gov't] 2007 Mar;50(4):453–464. - PubMed
- Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, et al. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clinical cancer research : an official journal of the American Association for Cancer Research. [Research Support, Non-U.S. Gov't] 2004 Apr 15;10(8):2790–2796. - PubMed
- Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005 Dec 15;11(24 Pt 1):8606–8614. - PubMed
- Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, et al. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jan 27;106(4):1193–1198. - PMC - PubMed
- Larue L, Beermann F. Cutaneous melanoma in genetically modified animals Pigment. Cell Res. 2007;20:485–497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous