The potential of GPNMB as novel neuroprotective factor in amyotrophic lateral sclerosis - PubMed (original) (raw)
doi: 10.1038/srep00573. Epub 2012 Aug 13.
Masamitsu Shimazawa, Masataka Kimura, Masafumi Takata, Kazuhiro Tsuruma, Mitsunori Yamada, Hitoshi Takahashi, Isao Hozumi, Jun-ichi Niwa, Yohei Iguchi, Takeshi Nikawa, Gen Sobue, Takashi Inuzuka, Hideaki Hara
Affiliations
- PMID: 22891158
- PMCID: PMC3417778
- DOI: 10.1038/srep00573
The potential of GPNMB as novel neuroprotective factor in amyotrophic lateral sclerosis
Hirotaka Tanaka et al. Sci Rep. 2012.
Abstract
Amyotrophic lateral sclerosis (ALS) is an incurable and fatal neurodegenerative disease characterized by the loss of motor neurons. Despite substantial research, the causes of ALS remain unclear. Glycoprotein nonmetastatic melanoma protein B (GPNMB) was identified as an ALS-related factor using DNA microarray analysis with mutant superoxide dismutase (SOD1(G93A)) mice. GPNMB was greatly induced in the spinal cords of ALS patients and a mouse model as the disease progressed. It was especially expressed in motor neurons and astrocytes. In an NSC34 cell line, glycosylation of GPNMB was inhibited by interaction with SOD1(G93A), increasing motor neuron vulnerability, whereas extracellular fragments of GPNMB secreted from activated astrocytes attenuated the neurotoxicity of SOD1(G93A) in neural cells. Furthermore, GPNMB expression was substantial in the sera of sporadic ALS patients than that of other diseased patients. This study suggests that GPNMB can be a target for therapeutic intervention for suppressing motor neuron degeneration in ALS.
Figures
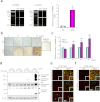
Figure 1. A progressive increase of glycoprotein nonmetastatic melanoma protein B (GPNMB) in the lumber spinal cords of mutant superoxide dismutase (SOD1G93A) mice.
(a) Quantitative real-time polymerase chain reaction reveals that GPNMB messenger RNA expression is enriched in the lumber spinal cords of 14-week-old SOD1G93A (G93A) mice. Values are mean ± SEM (n = 4). **P < 0.01 versus wild-type (WT) mice (Student's _t_-test). UM, upper marker; LM, lower marker. (b) Time-dependent increase in GPNMB in the lumber spinal cords of SOD1G93A mice. Scale bar = 50 μm. (c) Quantification of the density of GPNMB immunoreactivity in the lumber spinal cords of WT and SOD1G93A mice. Values are mean ± SEM (n = 3 to 5). **P < 0.01 versus WT mice (Student's _t_-test). ##P < 0.01 versus 6-week-old SOD1G93A mice (Dunnett's test). (d) Cleavage and up-regulation of GPNMB during disease progression in SOD1G93A mice determined using immunoblot analysis. (e, f) Enhanced GPNMB immunoreactivity in NeuN-positive motor neurons and glial fibrillary acidic protein-positive astrocytes, but not in ionized calcium binding adaptor molecule 1-positive microglia in the spinal cord gray (e) or white (f) matter of 14-week-old SOD1G93A mice. Scale bar = 50 μm.
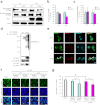
Figure 2. Downregulation of glycosylated GPNMB through interaction with SOD1G93A, leading to motor neuron death.
(a-c) NSC34 motor neuron cells were lysed after transfection with Myc-tagged WT SOD1 (SOD1wt) or SOD1G93A for 48 h. (a) Expression of GPNMB was examined using immunoblotting. The protein expression levels of glycosylated (b), but not non-glycosylated (c), GPNMB were decreased in the SOD1G93A-expressing cells. Values are mean ± SEM (n = 3 or 4). *P < 0.05 versus SOD1wt (Student's _t_-test). (d) Lysates from NSC34 cells transfected with Myc-tagged SOD1wt or SOD1G93A for 48 h were immunoprecipitated with an antibody to GPNMB (IP: GPNMB) and analyzed using immunoblotting with antibodies to ubiquitin, GPNMB, and Myc. (e) Laser scanning confocal photomicrographs of NSC34 cells showing the expression of GPNMB (green), ubiquitin (red), and Hoechst 33342 (blue). In the SOD1G93A-expressing cells, the aggregates of GPNMB in cytoplasm are partly colocalized with ubiquitin (arrowheads). Scale bar = 5 μm. (f, g) NSC34 cells were cotransfected with small interfering RNA (siRNA) against GPNMB or a nonspecific sequence (negative control) and enhanced green fluorescent protein-tagged mock, SOD1wt, or SOD1G93A for 48 h. Representative fluorescence microscopy showing nuclear staining for Hoechst 33342 (blue) and propidium iodide (red) (f). FBS, fetal bovine serum. The cell viability was reduced 48 h after the cotransfection of small interfering RNA and SOD1G93A (g). Values are mean ± SEM (n = 6). #P < 0.05 versus mock (Student's _t_-test). **P < 0.01 versus each negative control (Tukey's test). Scale bar = 100 μm.
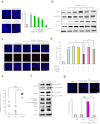
Figure 3. The extracellular fragments of GPNMB attenuated the neurotoxicity of SOD1G93A.
(a) The recombinant extracellular fragment of GPNMB at 0.25–2.5 µg/mL demonstrated a protective effect against SOD1G93A-induced cell death. Values are mean ± SEM (n = 3 to 6). #P < 0.05 versus control (Student's _t_-test). *P < 0.05, **P < 0.01 versus vehicle (Dunnett's test). Scale bar = 100 μm. (b) Time course of changes in phosphorylated ERK1/2 and phosphorylated Akt level after recombinant GPNMB treatment. (c, d) The protective effect of GPNMB against SOD1G93A-induced cell death was eliminated by LY294002, a PI3-kinase inhibitor, at 20 µM or by U0126, a MEK1/2 inhibitor, at 5 µM. Values are mean ± SEM (n = 6). ##P < 0.01 versus control (Student's _t_-test), **P < 0.01 versus SOD1G93A alone or SOD1G93A treated with GPNMB (Turkey's test). Scale bar = 100 μm. (e) Quantitative analysis of GPNMB in the conditioned media (CM) was performed using enzyme-linked immunosorbent assay. Values are mean ± SEM (n = 5). ##P < 0.01 versus mock, **P < 0.01 vs. SOD1wt (Tukey's test). (f) Expressions of GPNMB, MMP3, MMP9, and GFAP in NHA transfected with Myc-tagged SOD1wt or SOD1G93A were examined using immunoblotting. (g) CM from NHA transfected with SOD1G93A were immunoprecipitated with an antibody to GPNMB (IP: GPNMB) or control nonimmune antibody (IP: C) and added to NSC34 cells. Values are mean ± SEM (n = 5). ##P < 0.01 versus astrocyte basal medium, **P < 0.01 versus IP: C (Student's _t_-test). Scale bar = 100 μm.
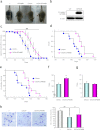
Figure 4. Regulation of amyotrophic lateral sclerosis (ALS) pathogenesis by GPNMB.
(a) Non-transgenic (−/−), GPNMB (-/GPNMB), SOD1G93A (G93A/-), and SOD1G93A/GPNMB double-transgenic (G93A/GPNMB) mice at approximately 5 weeks old. (b) The protein expression levels of V5-tagged GPNMB were increased in GPNMB-transgenic spinal cord tissues compared with those of the WT. (c) Motor performance assessed using the rotarod test for G93A/- and G93A/GPNMB mice. Values are mean ± SEM (n = 10). ##P < 0.01 versus G93A/- mice (two-way repeated measure ANOVA). *P < 0.05, **P < 0.01 versus G93A/- mice (Student's _t_-test). (d) Age of disease onset for G93A/- (n = 10) and G93A/GPNMB (n = 10) mice. Disease onset was defined at the day when a mouse first dropped off the rotarod within 600 s. P < 0.01 using the log-rank test. (e) Survival curve for G93A/- (n = 10) and G93A/GPNMB (n = 10) mice. P < 0.05 using the log-rank test. (f, g) Mean onset (f) and mean duration of disease progression (from onset to end stage; g) for G93A/- and G93A/GPNMB mice. Values are mean ± SEM (n = 10). **P < 0.01 versus G93A/- mice (Student's _t_-test). (h) Cresyl violet staining in the spinal cords of non-transgenic (−/−), SOD1G93A (G93A/-), and SOD1G93A/GPNMB double-transgenic (G93A/GPNMB) mice at 15 weeks old. Values are mean ± SEM (n = 3−5). ##P < 0.01 versus non-transgenic (−/−) mice, **P <0.01 versus SOD1G93A (G93A/-) mice. Scale bar = 25 μm.
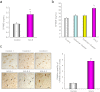
Figure 5. Representation of GPNMB protein in cerebrospinal fluid (CSF), sera, and lumber spinal cord tissues of sporadic ALS (SALS) patients.
(a, b) The amount of GPNMB secreted into CSF (a) or sera (b) in SALS patients was higher than that in controls and patients with Alzheimer disease and Parkinson disease. Values are mean ± SEM (n = 10 to 28). **P < 0.01 versus control (Mann–Whitney _U_-test; CSF samples). **P < 0.01 versus controls and patients with Alzheimer and Parkinson diseases (Tukey's test; sera samples). (c) Representative photographs are shown for the lumber spinal cords in the control and SALS patients. Extracellular deposition of GPNMB is observed in the lumber spinal cords of SALS (arrows). Scale bar = 100 μm. Values are mean ± SEM (n = 3). **P < 0.01 versus controls (Student's _t_-test).
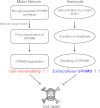
Figure 6. Hypothesized mechanisms for GPNMB regulation of motor neuron degeneration in ALS.
In motor neurons, glycosylation of GPNMB is inhibited by the interaction with SOD1G93A and GPNMB polyubiquitination. The downregulation of glycosylated GPNMB increases motor neuron vulnerability, ultimately triggering motor neuron death. Activated astrocytes secrete the extracellular fragments of GPNMB. The secretion of GPNMB is mediated by metalloproteinases such as a disintegrin and metalloproteinases and the fragments attenuate the neurotoxicity of SOD1G93A in motor neurons. Promoting the release of GPNMB extracellular fragments may rescue the motor neurons.
Similar articles
- Glycoprotein nonmetastatic melanoma protein B ameliorates skeletal muscle lesions in a SOD1G93A mouse model of amyotrophic lateral sclerosis.
Nagahara Y, Shimazawa M, Tanaka H, Ono Y, Noda Y, Ohuchi K, Tsuruma K, Katsuno M, Sobue G, Hara H. Nagahara Y, et al. J Neurosci Res. 2015 Oct;93(10):1552-66. doi: 10.1002/jnr.23619. Epub 2015 Jul 3. J Neurosci Res. 2015. PMID: 26140698 - GPNMB ameliorates mutant TDP-43-induced motor neuron cell death.
Nagahara Y, Shimazawa M, Ohuchi K, Ito J, Takahashi H, Tsuruma K, Kakita A, Hara H. Nagahara Y, et al. J Neurosci Res. 2017 Aug;95(8):1647-1665. doi: 10.1002/jnr.23999. Epub 2016 Dec 9. J Neurosci Res. 2017. PMID: 27935101 - The Potential Connection between Molecular Changes and Biomarkers Related to ALS and the Development and Regeneration of CNS.
Glavač D, Mladinić M, Ban J, Mazzone GL, Sámano C, Tomljanović I, Jezernik G, Ravnik-Glavač M. Glavač D, et al. Int J Mol Sci. 2022 Sep 26;23(19):11360. doi: 10.3390/ijms231911360. Int J Mol Sci. 2022. PMID: 36232667 Free PMC article. Review. - Glycoprotein non-metastatic melanoma protein B (GPNMB): An attractive target in atherosclerosis.
Yu X, Li M, Wang C, Guan X. Yu X, et al. Biochem Biophys Res Commun. 2024 Nov 5;732:150386. doi: 10.1016/j.bbrc.2024.150386. Epub 2024 Jul 15. Biochem Biophys Res Commun. 2024. PMID: 39024681 Review.
Cited by
- Long-term photoreceptor rescue in two rodent models of retinitis pigmentosa by adeno-associated virus delivery of Stanniocalcin-1.
Roddy GW, Yasumura D, Matthes MT, Alavi MV, Boye SL, Rosa RH Jr, Fautsch MP, Hauswirth WW, LaVail MM. Roddy GW, et al. Exp Eye Res. 2017 Dec;165:175-181. doi: 10.1016/j.exer.2017.09.011. Epub 2017 Sep 30. Exp Eye Res. 2017. PMID: 28974356 Free PMC article. - Genetic Determinants of Parkinson's Disease: Can They Help to Stratify the Patients Based on the Underlying Molecular Defect?
Redenšek S, Trošt M, Dolžan V. Redenšek S, et al. Front Aging Neurosci. 2017 Feb 10;9:20. doi: 10.3389/fnagi.2017.00020. eCollection 2017. Front Aging Neurosci. 2017. PMID: 28239348 Free PMC article. Review. - Tumor endothelial cell-induced CD8+ T-cell exhaustion via GPNMB in hepatocellular carcinoma.
Sakano Y, Noda T, Kobayashi S, Sasaki K, Iwagami Y, Yamada D, Tomimaru Y, Akita H, Gotoh K, Takahashi H, Asaoka T, Tanemura M, Wada H, Doki Y, Eguchi H. Sakano Y, et al. Cancer Sci. 2022 May;113(5):1625-1638. doi: 10.1111/cas.15331. Epub 2022 Mar 24. Cancer Sci. 2022. PMID: 35289033 Free PMC article. - Microglia express GPNMB in the brains of Alzheimer's disease and Nasu-Hakola disease.
Satoh JI, Kino Y, Yanaizu M, Ishida T, Saito Y. Satoh JI, et al. Intractable Rare Dis Res. 2019 May;8(2):120-128. doi: 10.5582/irdr.2019.01049. Intractable Rare Dis Res. 2019. PMID: 31218162 Free PMC article.
References
- Dion P. A., Daoud H. & Rouleau G. A. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 10, 769–782 (2009). - PubMed
- Jackson M., Ganel R. & Rothstein J. D. Models of amyotrophic lateral sclerosis. Curr Protoc Neurosci. Chapter 9, Unit 9 13 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous