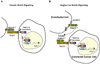Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1 - PubMed (original) (raw)
. 2013 Feb 11;23(2):171-85.
doi: 10.1016/j.ccr.2012.12.021. Epub 2013 Jan 31.
Xiangcang Ye, Fan Fan, Ling Xia, Rajat Bhattacharya, Seth Bellister, Federico Tozzi, Eric Sceusi, Yunfei Zhou, Isamu Tachibana, Dipen M Maru, David H Hawke, Janusz Rak, Sendurai A Mani, Patrick Zweidler-McKay, Lee M Ellis
Affiliations
- PMID: 23375636
- PMCID: PMC3574187
- DOI: 10.1016/j.ccr.2012.12.021
Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1
Jia Lu et al. Cancer Cell. 2013.
Abstract
We report a paracrine effect whereby endothelial cells (ECs) promote the cancer stem cell (CSC) phenotype of human colorectal cancer (CRC) cells. We showed that, without direct cell-cell contact, ECs secrete factors that promoted the CSC phenotype in CRC cells via Notch activation. In human CRC specimens, CD133 and Notch intracellular domain-positive CRC cells colocalized in perivascular regions. An EC-derived, soluble form of Jagged-1, via ADAM17 proteolytic activity, led to Notch activation in CRC cells in a paracrine manner; these effects were blocked by immunodepletion of Jagged-1 in EC-conditioned medium or blockade of ADAM17 activity. Collectively, ECs play an active role in promoting Notch signaling and the CSC phenotype by secreting soluble Jagged-1.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Figure 1. Endothelial Cells Promote the CSC Phenotype in CRC Cells in vitro
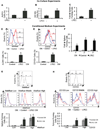
(A) Freshly isolated xenografted human CRC (xhCRC) cells were co-cultured with freshly isolated human liver parenchyma endothelial cells (LPECs) and the xhCRC Aldefluor-positive cell population was determined. (B) Freshly isolated xhCRC cells were co-cultured with freshly isolated LPECs and the xhCRC CD133-positive cell population was determined. (C) Freshly isolated xhCRC cells were co-cultured with freshly isolated LPECs and the xhCRC sphere forming assay was performed. (D) Freshly isolated human CRC (hCRC) cells were treated with LPEC-derived conditioned medium (CM), and the Aldefluor-positive cell population was determined. (E) Freshly isolated human CRC (hCRC) cells were treated with LPEC-derived conditioned medium (CM), and the CD133-positive cell population was determined. (F) Sphere-forming capability in 1°, 2°, and 3° cultures of freshly isolated hCRC cells treated with LPEC CM. (G) xhCRC cells were FAC-sorted by the Aldefluor assay into high, middle, and low cell populations (upper panel). The Aldefluor-high, -middle, and -low cell populations were treated with LPEC CM in parallel (middle panel); the Aldefluor assay was then repeated to determine which ALDH fraction could be enriched for CSCs by EC CM (lower panel). (H) xhCRC cells were FAC-sorted for high, middle, and low CD133 expression (upper panel). The CD133-high, -middle, and -low fractions were treated with LPEC CM in parallel (middle panel); the flow cytometry analysis on CD133 was then repeated to determine which CD133 fraction could be enriched for CSCs by EC CM (lower panel). *p<0.05, mean ± SEM. See also Figure S1.
Figure 2. Endothelial Cells Promote the CSC Phenotype of CRC Cells in vivo
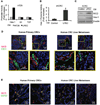
(A) In vivo tumorigenicity assay with limited dilution; HT29 cells pretreated with control CM or LPEC CM. (B) In vivo tumorigenicity assay with limited dilution combined with serial transplantation using freshly isolated xhCRC cells pretreated with control CM or LPEC CM. (C/D) Hepatic metastatic incidence and burden of xhCRC cells pre-treated with control CM or LPEC CM in a splenic injection model (*p<0.05, mean ± SEM). (E) Representative immunofluorescent staining of CD133 and CD31 in human primary CRC surgical specimens (left panel), and human CRC liver metastases (right panel). The highlighted region in the upper panel is enlarged in the lower panel. See also Figure S2.
Figure 3. Endothelial Cell Conditioned Medium Promotes Chemoresistance of Colorectal Cancer Cells
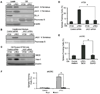
(A) HCT116 cells treated with oxaliplatin and 5-FU simultaneous to the addition of control CM or RF24 CM (MTT assay). (B) Western blot analysis of the expression of pro-apoptotic markers in HCT116 cells treated with oxaliplatin and 5-FU simultaneous to the addition of control CM or RF24 CM. (C) xhCRC cells treated with oxaliplatin and 5-FU simultaneous to the addition of control CM or LPEC CM (MTT assay). (D) Western blot analysis of the expression of pro-apoptotic markers in xhCRC cells treated with 5-FU simultaneous to the addition of control CM or LPEC CM. *p<0.05, mean ± SEM.
Figure 4. Endothelial Cells Activate Notch Signaling in Neighboring Colorectal Cancer Cells
(A) Promoter activity of Hes-1, Gli, and TCF in HT29 cells after treatment with control CM or LPEC CM. (B) Hes-1 promoter activity in freshly isolated xhCRC cells treated with control CM or LPEC CM (*p<0.05, mean ± SEM). (C) NICD and Hes-1 expression of freshly isolated hCRC cells treated with control CM or LPEC CM. (D) Representative immunofluorescent staining for NICD and CD31 in human primary CRCs (left panel) and CRC liver metastases (right panel). The highlighted region in the upper panel is enlarged in the lower panel. (E) Representative immunofluorescent staining of NICD and CD133 in primary CRCs (left panel) and CRC liver metastases (right panel). See also Figure S3.
Figure 5. Endothelial Cells Secrete a Soluble Form of Jagged-1 to Promote the CSC Phenotype in Colorectal Cancer Cells
(A) Western blot analysis of HT29 and LPEC cell lysates and conditioned medium utilizing antibodies to the N-terminus and C-terminus regions of Jagged-1 (JAG1). Western blotting for DLL4 was also performed. (B) Detection of soluble Jagged-1 in the conditioned medium from HT29 cells and LPECs after siRNA-mediated Jagged-1 knockdown. (C) NICD and Hes-1 expression in HT29 cells after treatment with control CM or LPEC CM with decreased Jagged-1 levels by siRNA knockdown. (D) Sphere forming assay of HT29 cells exposed to control CM or LPEC CM with decreased Jagged-1 levels by siRNA knockdown. (E) Sphere forming assay of freshly isolated xhCRC cells exposed to control CM or LPEC CM with decreased Jagged-1 levels by siRNA knockdown. (F) Sphere forming assay of freshly isolated xhCRC cells exposed to the LPEC CM that is Jagged-1 depleted by immunoprecipitation. See also Figure S4.
Figure 6. Proteomics Analysis Demonstrate that the EC-secreted Soluble Form of Jagged-1 is C-terminally Truncated by ADAM17
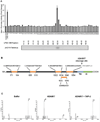
(A) Concentrated LPEC CM was fractionated by FPLC gel filtration. The fractions were applied to xhCRC cells containing the Hes-1 promoter - luciferase construct, and Hes-1 promoter activity was assessed (upper panel). Western blot detection of soluble Jagged-1 in combined adjacent fractions is shown in the lower panel. (B) Jagged-1 from LPEC CM was immunoprecipitated using an N-terminal antibody and subjected them to deglycosylation followed by digestion. Mass spectrometric analysis of Jagged-1 proteins was performed with multiple peptides that identified consistent with the N-terminal region of Jagged-1 with a C-terminus at amino acid 1054. (C) Mass spectrometric analysis of the ADAM17 cleavage site of Jagged-1. A synthetic peptide corresponding to aa1047–1061 of Jagged-1 was incubated with buffer only (left panel). ADAM17 (middle panel), or ADAM17 with its inhibitor TAPI-2 (right panel), and the reaction products were subjected to mass spectrometry. Peak 1 represents the intact substrate SLIAAVAEVRVQRRP, and Peak 2 represents the ADAM17 cleavage product, a 906.56 Da peptide that was determined to be VRVQRRP. See also S5.
Figure 7. Inhibition of ADAM17 in Endothelial Cells Blocks the Conditioned Medium Promotion of the CSC Phenotype in Human CRC Cells
(A) Western blot detection of ADAM17 in the cell lysate of LPECs or LPECs with decreased ADAM17 by siRNA knockdown (left panel). The soluble form of Jagged-1 in the CM from the cells shown in the left panel, are shown in the right panel. (B) Secretion of the soluble form of Jagged-1 into the CM of HT29 cells and LPECs treated with the ADAM17 inhibitor TAPI-2. (C/D) Sphere forming assays on HT29 cells (Panel C) and freshly isolated xhCRC cells (Panel D) after treatment with CM from LPECs, with or without the ADAM17 inhibitor TAPI-2 (*p<0.05, mean ± SEM). (E) In vivo tumorigenicity assay (day 10) using freshly isolated xhCRC cells co-injected with LPECs with/without daily TAPI-2 treatment (*p<0.05, LPEC/no TAPI-2 vs all other groups). See also S6.
Figure 8. Proposed Model for EC-mediated Paracrine Activation of Notch Signaling in Colorectal Cancer Cells
(A) The conical model for Notch pathway activation, where membrane bound ligands such as Jagged-1 activates Notch signaling in contacting cells. (B) Schematic summarizing our proposed model for paracrine activation of the Notch pathway in CRC cells. ADAM17 cleaves membrane bound Jagged-1 on ECs, releasing an N-terminal soluble fragment that binds, and activates, Notch on CRC cells.
Similar articles
- The wnt target jagged-1 mediates the activation of notch signaling by progastrin in human colorectal cancer cells.
Pannequin J, Bonnans C, Delaunay N, Ryan J, Bourgaux JF, Joubert D, Hollande F. Pannequin J, et al. Cancer Res. 2009 Aug 1;69(15):6065-73. doi: 10.1158/0008-5472.CAN-08-2409. Epub 2009 Jul 21. Cancer Res. 2009. PMID: 19622776 - Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain.
Xing F, Kobayashi A, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Wilber A, Mo YY, Moore BE, Liu W, Fukuda K, Iiizumi M, Sharma S, Liu Y, Wu K, Peralta E, Watabe K. Xing F, et al. EMBO Mol Med. 2013 Mar;5(3):384-96. doi: 10.1002/emmm.201201623. EMBO Mol Med. 2013. PMID: 23495140 Free PMC article. - Silencing of Jagged1 inhibits cell growth and invasion in colorectal cancer.
Dai Y, Wilson G, Huang B, Peng M, Teng G, Zhang D, Zhang R, Ebert MP, Chen J, Wong BC, Chan KW, George J, Qiao L. Dai Y, et al. Cell Death Dis. 2014 Apr 10;5(4):e1170. doi: 10.1038/cddis.2014.137. Cell Death Dis. 2014. PMID: 24722295 Free PMC article. - Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.
Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu M, Ye S, Liu Y. Chen X, et al. Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review. - Epigenetic silencing of Notch signaling in gastrointestinal cancers.
Piazzi G, Bazzoli F, Ricciardiello L. Piazzi G, et al. Cell Cycle. 2012 Dec 1;11(23):4323-7. doi: 10.4161/cc.22388. Epub 2012 Oct 19. Cell Cycle. 2012. PMID: 23085543 Free PMC article. Review.
Cited by
- Jagged mediates differences in normal and tumor angiogenesis by affecting tip-stalk fate decision.
Boareto M, Jolly MK, Ben-Jacob E, Onuchic JN. Boareto M, et al. Proc Natl Acad Sci U S A. 2015 Jul 21;112(29):E3836-44. doi: 10.1073/pnas.1511814112. Epub 2015 Jul 7. Proc Natl Acad Sci U S A. 2015. PMID: 26153421 Free PMC article. - Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer.
Welti J, Loges S, Dimmeler S, Carmeliet P. Welti J, et al. J Clin Invest. 2013 Aug;123(8):3190-200. doi: 10.1172/JCI70212. Epub 2013 Aug 1. J Clin Invest. 2013. PMID: 23908119 Free PMC article. Review. - Reprogramming endothelial cells to empower cancer immunotherapy.
Cleveland AH, Fan Y. Cleveland AH, et al. Trends Mol Med. 2024 Feb;30(2):126-135. doi: 10.1016/j.molmed.2023.11.002. Epub 2023 Nov 30. Trends Mol Med. 2024. PMID: 38040601 Review. - Liver Endothelium Promotes HER3-Mediated Cell Survival in Colorectal Cancer with Wild-Type and Mutant KRAS.
Rathore M, Zhang W, Wright M, Bhattacharya R, Fan F, Vaziri-Gohar A, Winter J, Wang Z, Markowitz SD, Willis J, Ellis LM, Wang R. Rathore M, et al. Mol Cancer Res. 2022 Jun 3;20(6):996-1008. doi: 10.1158/1541-7786.MCR-21-0633. Mol Cancer Res. 2022. PMID: 35276002 Free PMC article. - The Prognostic Role and Significance of Dll4 and Toll-like Receptors in Cancer Development.
Fasoulakis Z, Koutras A, Ntounis T, Pergialiotis V, Chionis A, Katrachouras A, Palios VC, Symeonidis P, Valsamaki A, Syllaios A, Diakosavvas M, Angelou K, Samara AA, Pagkalos A, Theodora M, Schizas D, Kontomanolis EN. Fasoulakis Z, et al. Cancers (Basel). 2022 Mar 24;14(7):1649. doi: 10.3390/cancers14071649. Cancers (Basel). 2022. PMID: 35406423 Free PMC article. Review.
References
- ACS. Cancer Statistic 2010. 2010 http://wwwcancerorg/research/cancerfactsfigures/cancerfactsfigures/cance....
- Aho S. Soluble form of Jagged1: unique product of epithelial keratinocytes and a regulator of keratinocyte differentiation. J Cell Biochem. 2004;92:1271–1281. - PubMed
- Al-Hajj M. Cancer stem cells and oncology therapeutics. Curr Opin Oncol. 2007;19:61–64. - PubMed
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- R01 CA157880/CA/NCI NIH HHS/United States
- T32 CA009599/CA/NCI NIH HHS/United States
- T32CA009599/CA/NCI NIH HHS/United States
- CA100879/CA/NCI NIH HHS/United States
- R01CA157880/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous