Integrated transcriptomic and epigenomic analysis of primary human lung epithelial cell differentiation - PubMed (original) (raw)
. 2013 Jun;9(6):e1003513.
doi: 10.1371/journal.pgen.1003513. Epub 2013 Jun 20.
Beiyun Zhou, Megan E Rieger, Suhaida A Selamat, Mickael Dubourd, Xiaohui Fang, Sean K Lynch, Theresa Ryan Stueve, Kimberly D Siegmund, Benjamin P Berman, Zea Borok, Ite A Laird-Offringa
Affiliations
- PMID: 23818859
- PMCID: PMC3688557
- DOI: 10.1371/journal.pgen.1003513
Integrated transcriptomic and epigenomic analysis of primary human lung epithelial cell differentiation
Crystal N Marconett et al. PLoS Genet. 2013 Jun.
Abstract
Elucidation of the epigenetic basis for cell-type specific gene regulation is key to gaining a full understanding of how the distinct phenotypes of differentiated cells are achieved and maintained. Here we examined how epigenetic changes are integrated with transcriptional activation to determine cell phenotype during differentiation. We performed epigenomic profiling in conjunction with transcriptomic profiling using in vitro differentiation of human primary alveolar epithelial cells (AEC). This model recapitulates an in vivo process in which AEC transition from one differentiated cell type to another during regeneration following lung injury. Interrogation of histone marks over time revealed enrichment of specific transcription factor binding motifs within regions of changing chromatin structure. Cross-referencing of these motifs with pathways showing transcriptional changes revealed known regulatory pathways of distal alveolar differentiation, such as the WNT and transforming growth factor beta (TGFB) pathways, and putative novel regulators of adult AEC differentiation including hepatocyte nuclear factor 4 alpha (HNF4A), and the retinoid X receptor (RXR) signaling pathways. Inhibition of the RXR pathway confirmed its functional relevance for alveolar differentiation. Our incorporation of epigenetic data allowed specific identification of transcription factors that are potential direct upstream regulators of the differentiation process, demonstrating the power of this approach. Integration of epigenomic data with transcriptomic profiling has broad application for the identification of regulatory pathways in other models of differentiation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
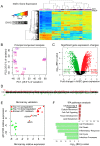
Figure 1. Transcriptomic profiling of human AEC differentiation.
A) Heatmap of top 5% variant-VSN normalized gene expression probes. Blue = low expression, red = high expression. DAY = number of days AT2 cells were allowed to differentiate. “Prep” = donor lung origin by color (Figure S1). B) Principal component analysis of normalized hAEC samples. Samples color coded by donor lung as in (A). C) Significant changes in hAEC gene expression. Black line = BH-adjusted cutoff (FDR adjusted p≤0.05) calculated between D0 and D8. 20 genes show both significant up and downregulation for probes in different locations of the gene. D) Manhattan plot of differentially expressed genes. X-axis = chromosomal location, Y-axis = number of genes in each 2 MB region. E) qRT-PCR validation of microarray, data expressed in log2-fold change of differences between D0 and D8. Circles = top 10 up- and down-regulated genes, triangles = known AT1 cell differentiation markers (AQP5, PDPN, CAV1). F) IPA of significantly up- or down-regulated genes. Bars expressed as log10-BH corrected p-values of enrichment for pathway members in significant list against RefSeq db38 background. Whole figure: Red = upregulated, green = downregulated.
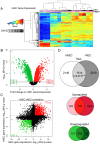
Figure 2. Comparative transcriptomic profiling of human and rat AEC differentiation.
A) Heatmap of top 2% of variant-VSN normalized gene expression probes in rat AEC. Blue = low expression, red = high expression. Prep = separate rAEC purifications. B) Significant changes in rAEC gene expression: red = upregulated, green = downregulated, black line = BH-adjusted cutoff for significance (FDR adjusted p≤0.05) calculated between D0 and D8. C) Correlation between rAEC and hAEC statistically significant genes. Data points expressed as significance of change between D0 and D8. Direction of change derived from increase or decrease in gene expression. Red = statically significant upregulated genes in both hAEC and rAEC, green = statistically significant downregulated genes in both hAEC and rAEC. Dotted lines = BH-adjusted cutoff for significance (p.adjusted≤0.05) calculated between D0 and D8. D) Venn diagram of statistically significant gene overlap between hAEC and rAEC (Top), genes upregulated between hAEC and rAEC (Middle), and genes downregulated between hAEC and rAEC (Bottom). 271 genes were significant in both species but expression changed in opposite directions. In all three diagrams: pale color = hAEC-specific statistically significant gene expression changes, medium color = statistically significant overlap in both rAEC and hAEC, dark color = rAEC-specific statistically significant gene expression changes.
Figure 3. Chromatin changes during AEC differentiation.
A) Manhattan plot of differential chromatin changes. X-axis = chromosomal location, Y-axis = number of cell type-specific chromatin changes within 2 MB region. Upper panel = H3K9/14Ac changes, blue = AT2 cell-specific acetylation, purple = AT1 cell-specific acetylation. Lower panel = H3K27me3 changes, orange = AT2 cell-specific methylation, grey = AT1 cell-specific methylation. B) 135 TFBS enrichment in domains of chromatin change from HOMER. X-axis = H3K9/14Ac, Y-axis = H3K27me3 enrichment. AT2 enrichment is shown as the log10 TFBS p-value, AT1 enrichment is shown as the −log10 TFBS p-value. C) Example of chromatin changes at an upregulated gene, FZD2, using IGV to visualize chromatin tracks. Blue = H3K9/14Ac raw reads and SICER peaks called, green = predicted RXR binding site from HOMER analysis. D) Example of downregulated gene expression at the PGC gene locus. Lavender = predicted FOXA1 binding sites from HOMER analysis. AT2 = AEC chromatin signature (D0), AT1 = AEC chromatin signature (D8).
Figure 4. Integration of gene expression data with epigenetic alterations.
A–B–C) Relationship between all 16 possible combinations of chromatin changes and gene expression. Grey = unassociated with H3K9/14Ac or H3K27me3 changes, red = potentially activating chromatin changes, green = potentially repressive chromatin changes, blue = mixture of both. A) Significant expression changes in genes as a percentage of all genes associated with each histone mark for each of the possible 16 combinations of chromatin marks. Upregulated = above x-axis, downregulated = below x-axis. B) Total number of genes with significant gene expression changes associated with each chromatin combination. C) Representative IPA network of upregulated genes with both H3K9/14Ac gain and H3K27me3 loss. D) Representative IPA network of downregulated genes with H3K9/14Ac loss. E and F) IPA ranked networks of genes subset by chromatin context. Corresponding TFBS present in subset chromatin and enrichment p-value from HOMER analysis, for each chromatin-associated gene subset. Red = upregulated gene expression and activating chromatin changes, green = downregulated gene expression and deactivating chromatin changes. (*) Indicates below significance threshold in HOMER but still present in IPA.
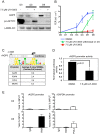
Figure 5. Functional validation of a transcription factor signaling pathway predicted from bioinformatics analysis.
A) Western blots examining AT2 and AT1 cell markers during differentiation in the presence or absence of RXR antagonist UVI-3003. LAMIN A/C is the loading control. B) Transepithelial resistance as measured in kΩ-cm2 over the course of differentiation. Error bars represent technical duplicates for each plating. C) Rat _Aqp5_-luciferase 4.3 kb promoter construct. Grey lines = 34 putative PPARA:RXR binding sites (Explain3.0). No sites were predicted from −900 to +6 bp due to lack of rat sequence information in the Explain v3.0 database. The asterisk marks the approximate location in the promoter of the ChIPed RXR site in E, below. The average number of PPARA:RXR sites per kilobase in the listed human/rat/mouse promoters is given in the table, with consensus site listed at the top. D) MLE-15 cells were transiently transfected with the _Aqp5_-luciferase construct and treated for 48 hours with vehicle (DMSO) or 7.5 µM UVI-3003. UV1-3003 treatment reduced _Aqp5_-luc activity by 48%±0.06. Values were normalized to vehicle control and represent the mean, error bars represent SEM, N = 3. All experiments represent 3 biological replicates. E) ChIP was performed on primary cultured rat AEC at day 0 (AT2, D0, n = 2) and day 8 (AT1-like, D8, n = 3). A region ∼4 kb upstream of the transcription start site specifically precipitated with RXR in day 8 samples. ChIP of GAPDH with RXR was performed as a control, and POL2 (POLR2A) binding to the GAPDH promoter was included as a positive control for the quality of day 0 DNA.
Similar articles
- Comprehensive epigenomic profiling of human alveolar epithelial differentiation identifies key epigenetic states and transcription factor co-regulatory networks for maintenance of distal lung identity.
Zhou B, Stueve TR, Mihalakakos EA, Miao L, Mullen D, Wang Y, Liu Y, Luo J, Tran E, Siegmund KD, Lynch SK, Ryan AL, Offringa IA, Borok Z, Marconett CN. Zhou B, et al. BMC Genomics. 2021 Dec 18;22(1):906. doi: 10.1186/s12864-021-08152-6. BMC Genomics. 2021. PMID: 34922464 Free PMC article. - Transcriptomic Profiling of Primary Alveolar Epithelial Cell Differentiation in Human and Rat.
Marconett CN, Zhou B, Siegmund KD, Borok Z, Laird-Offringa IA. Marconett CN, et al. Genom Data. 2014 Dec 1;2:105-109. doi: 10.1016/j.gdata.2014.05.011. Genom Data. 2014. PMID: 25343132 Free PMC article. - Commitment and differentiation of lung cell lineages.
Warburton D, Wuenschell C, Flores-Delgado G, Anderson K. Warburton D, et al. Biochem Cell Biol. 1998;76(6):971-95. Biochem Cell Biol. 1998. PMID: 10392710 Review. - Integrative epigenomic analysis in differentiated human primary bronchial epithelial cells exposed to cigarette smoke.
Glass K, Thibault D, Guo F, Mitchel JA, Pham B, Qiu W, Li Y, Jiang Z, Castaldi PJ, Silverman EK, Raby B, Park JA, Yuan GC, Zhou X. Glass K, et al. Sci Rep. 2018 Aug 24;8(1):12750. doi: 10.1038/s41598-018-30781-3. Sci Rep. 2018. PMID: 30143676 Free PMC article. - Transcriptional and Epigenomic Regulation of Adipogenesis.
Lee JE, Schmidt H, Lai B, Ge K. Lee JE, et al. Mol Cell Biol. 2019 May 14;39(11):e00601-18. doi: 10.1128/MCB.00601-18. Print 2019 Jun 1. Mol Cell Biol. 2019. PMID: 30936246 Free PMC article. Review.
Cited by
- WNT signaling - lung cancer is no exception.
Rapp J, Jaromi L, Kvell K, Miskei G, Pongracz JE. Rapp J, et al. Respir Res. 2017 Sep 5;18(1):167. doi: 10.1186/s12931-017-0650-6. Respir Res. 2017. PMID: 28870231 Free PMC article. Review. - Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis.
Lehmann M, Buhl L, Alsafadi HN, Klee S, Hermann S, Mutze K, Ota C, Lindner M, Behr J, Hilgendorff A, Wagner DE, Königshoff M. Lehmann M, et al. Respir Res. 2018 Sep 15;19(1):175. doi: 10.1186/s12931-018-0876-y. Respir Res. 2018. PMID: 30219058 Free PMC article. - Knockout mice reveal key roles for claudin 18 in alveolar barrier properties and fluid homeostasis.
Li G, Flodby P, Luo J, Kage H, Sipos A, Gao D, Ji Y, Beard LL, Marconett CN, DeMaio L, Kim YH, Kim KJ, Laird-Offringa IA, Minoo P, Liebler JM, Zhou B, Crandall ED, Borok Z. Li G, et al. Am J Respir Cell Mol Biol. 2014 Aug;51(2):210-22. doi: 10.1165/rcmb.2013-0353OC. Am J Respir Cell Mol Biol. 2014. PMID: 24588076 Free PMC article. - Coactivator-Associated Arginine Methyltransferase-1 Function in Alveolar Epithelial Senescence and Elastase-Induced Emphysema Susceptibility.
Sarker RS, John-Schuster G, Bohla A, Mutze K, Burgstaller G, Bedford MT, Königshoff M, Eickelberg O, Yildirim AÖ. Sarker RS, et al. Am J Respir Cell Mol Biol. 2015 Dec;53(6):769-81. doi: 10.1165/rcmb.2014-0216OC. Am J Respir Cell Mol Biol. 2015. PMID: 25906418 Free PMC article. - TENET 2.0: Identification of key transcriptional regulators and enhancers in lung adenocarcinoma.
Mullen DJ, Yan C, Kang DS, Zhou B, Borok Z, Marconett CN, Farnham PJ, Offringa IA, Rhie SK. Mullen DJ, et al. PLoS Genet. 2020 Sep 14;16(9):e1009023. doi: 10.1371/journal.pgen.1009023. eCollection 2020 Sep. PLoS Genet. 2020. PMID: 32925947 Free PMC article.
References
- Vasanthi D, Mishra RK (2008) Epigenetic regulation of genes during development: a conserved theme from flies to mammals. J Genet Genomics 35: 413–429. - PubMed
- Bernstein BE, Meissner A, Lander ES (2007) The mammalian epigenome. Cell 128: 669–681. - PubMed
- Hirschhorn JN, Brown SA, Clark CD, Winston F (1992) Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes Dev 6: 2288–2298. - PubMed
Publication types
MeSH terms
Grants and funding
- 1 P30 H101258/PHS HHS/United States
- R37HL062569-13/HL/NHLBI NIH HHS/United States
- P30CA014089/CA/NCI NIH HHS/United States
- R01 HL114094/HL/NHLBI NIH HHS/United States
- 1 R01 HL114094/HL/NHLBI NIH HHS/United States
- P30 CA014089/CA/NCI NIH HHS/United States
- R01 HL112638/HL/NHLBI NIH HHS/United States
- T32 ES013678/ES/NIEHS NIH HHS/United States
- R37 HL062569/HL/NHLBI NIH HHS/United States
- PFTED-10-207-01-SIED/PHS HHS/United States
- T32 CA009320/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases