Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice - PubMed (original) (raw)
Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice
Bhanu Priya Ganesh et al. PLoS One. 2013.
Abstract
Excessive mucin degradation by intestinal bacteria may contribute to inflammatory bowel diseases because access of luminal antigens to the intestinal immune system is facilitated. This study investigated how the presence of a mucin degrading commensal bacterium affects the severity of an intestinal Salmonella enterica Typhimurium-induced gut inflammation. Using a gnotobiotic C3H mouse model with a background microbiota of eight bacterial species (SIHUMI) the impact of the mucin-degrading commensal bacterium Akkermansia muciniphila (SIHUMI-A) on inflammatory and infectious symptoms caused by S. Typhimurium was investigated. Presence of A. muciniphila in S. Typhimurium-infected SIHUMI mice caused significantly increased histopathology scores and elevated mRNA levels of IFN-γ, IP-10, TNF-α, IL-12, IL-17 and IL-6 in cecal and colonic tissue. The increase in pro-inflammatory cytokines was accompanied by 10-fold higher S. Typhimurium cell numbers in mesenteric lymph nodes of SIHUMI mice associated with A. muciniphila and S. Typhimurium (SIHUMI-AS) compared to SIHUMI mice with S. Typhimurium only (SIHUMI-S). The number of mucin filled goblet cells was 2- to 3-fold lower in cecal tissue of SIHUMI-AS mice compared to SIHUMI-S, SIHUMI-A or SIHUMI mice. Reduced goblet cell numbers significantly correlated with increased IFN-γ mRNA levels (r(2) = -0.86, ***P<0.001) in all infected mice. In addition, loss of cecal mucin sulphation was observed in SIHUMI mice containing both A. muciniphila and S. Typhimurium compared to other mouse groups. Concomitant presence of A. muciniphila and S. Typhimurium resulted in a drastic change in microbiota composition of SIHUMI mice: the proportion of B. thetaiotaomicron in SIHUMI-AS mice was 0.02% of total bacteria compared to 78%-88% in the other mouse groups and the proportion of S. Typhimurium was 94% in SIHUMI-AS mice but only 2.2% in the SIHUMI-S mice. These results indicate that A. muciniphila exacerbates S. Typhimurium-induced intestinal inflammation by its ability to disturb host mucus homeostasis.
Conflict of interest statement
Competing Interests: Corresponding author (Gunnar Loh) is a PLOS ONE Editorial Board member. This does not alter the authors' adherence to all PLOS ONE policies on sharing data and material.
Figures
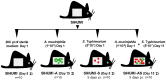
Figure 1. Design of the animal experiment.
Fourty C3H mice associated with a defined microbial community of 8 bacterial species (SIHUMI) were allocated to four different groups (10 mice per group). Each mouse was associated with 8 bacterial species (SIHUMI). Twelve weeks-old SIHUMI mice were subsequently associated with A. muciniphila (SIHUMI-A) or S. Typhimurium (SIHUMI-S) or with both A. muciniphila and S. Typhimurium (SIHUMI-AS). SIHUMI mice received only sterile medium. Times of association, infection and killing are as indicated. ‡ - killed.
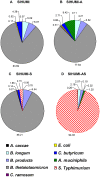
Figure 2. Presence of A. muciniphila renders S. Typhimurium the dominant species in gnotobiotic SIHUMI mice.
Cecal contents were collected from gnotobiotic C3H mice, differing in their microbial status: (A) Mice with a defined microbial community of eight bacterial species (SIHUMI), (B) SIHUMI mice additionally colonized with A. muciniphila (SIHUMI-A), (C) SIHUMI mice infected with S. Typhimurium (SIHUMI-S) and (D) SIHUMI mice colonized with A. muciniphila and 10 days later infected with S. Typhimurium (SIHUMI-AS) (see Figure 1). Total DNA was extracted and bacterial cell numbers were quantified by qPCR with primers targeting the HSP60 gene of the SIHUMI members, the 16S rRNA gene of A. muciniphila and the ttr-region of S. Typhimurium. Calculation of the cell numbers was based on DNA obtained from cell suspensions containing known cell numbers of the targeted bacterial species (see materials and methods). Presence of A. muciniphila in SIHUMI-AS mice is attributed to an increase in the proportion of S. Typhimurium cells at the expense of other community members showing reduced proportion of SIHUMI members. Ten animals per group were used. The bacterial cell numbers and P-values for the differences between the groups are provided in Table 1.
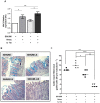
Figure 3. Concomitant presence of A. muciniphila and S. Typhimurium results in increased histopathology scores in SIHUMI mice.
(A) Gnotobiotic C3H mice containing 8 defined microbial species (SIHUMI) were subsequently inoculated with A. muciniphila or S. Typhimurium or consecutively with both organisms (see Figure 1). SIHUMI and SIHUMI-A mice had the lowest histopathology scores (≤4.0) with no signs of inflammation and were therefore taken as baseline (dotted line). Data are expressed as median with range. *P<0.05, **P<0.01, ***P<0.001. n = 10 mice per group. (B) Representative microscopy images of pathological changes observed in cecum tissue sections fixed with formalin and stained with hematoxylin and eosin (4 µm) of the four mouse groups. n = 10 mice per group; Magnification: 1000-fold.
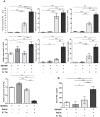
Figure 4. Presence of both A. muciniphila and S. Typhimurium is accompanied by increased pro-inflammatory cytokines.
(A) Cecal mRNA levels of IFN-γ, IP-10, TNF-α, IL-12, IL-6, IL-17 and IL-18 in gnotobiotic SIHUMI mice were measured. mRNA was extracted from cecum mucosa of mice belonging to either one of four groups: SIHUMI, SIHUMI-A, SIHUMI-S and SIHUMI-AS (see Figure. 1). The mRNA was converted to cDNA for quantitative real-time PCR measurement (see materials and methods). Inoculation of the gnotobiotic SIHUMI mice with A. muciniphila followed by S. Typhimurium infection (SIHUMI-AS) caused an increase in mRNA levels of pro-inflammatory cytokines except IL-18. Data are expressed as mean±standard error. n = 6 per group. Star indicates statistically significant differences (*P<0.05, **P<0.01, ***P<0.001). AU: Arbitrary units; Amuc: A. muciniphila; S. Tm: S. Typhimurium. (B) Serum protein levels of IFN-γ were increased in SIHUMI-AS mice compared to the other mouse groups. Data are expressed as mean±standard error. n = 10 mice per group. *P<0.05, **P<0.01, ***P<0.001. Amuc: A. muciniphila; S. Tm: S. Typhimurium.
Figure 5. SIHUMI mice colonized with both A. muciniphila and S. Typhimurium display enlarged mLN and elevated S. Typhimurium cell numbers.
(A) Mesenteric lymph nodes (mLN) were obtained from four groups of gnotobiotic C3H mice. SIHUMI mice were subsequently inoculated with A. muciniphila or S. Typhimurium or consecutively with both organisms (see Figure 1). The mLN tissue was homogenized and DNA was isolated to quantify S. Typhimurium using quantitative PCR with primers targeting the ttr-region of S. Typhimurium. Absolute cell numbers were calculated based on calibration curves with known concentrations of S. Typhimurium. The mLN of SIHUMI-AS mice contained 10-fold higher cell numbers of S. Typhimurium compared to SIHUMI-S mice. Data are expressed as mean±standard error. n = 10 mice per group. *P<0.05, **P<0.01, ***P<0.001. n.d: not detected. Amuc: A. muciniphila; S. Tm: S. Typhimurium. (B) The photograph shows four lymph nodes, each representative of one of the four mouse groups and a cm scale. Twelve week old gnotobiotic SIHUMI mice with both A. muciniphila and S. Typhimurium displayed an increased size of their mesenteric lymph nodes compared to SIHUMI mice infected with S. Typhimurium only.
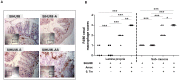
Figure 6. SIHUMI mice colonized with both A. muciniphila and S. Typhimurium display an increased cecal macrophage infiltration.
(A) Formalin fixed paraffin embedded cecum tissue was thin sectioned at 2 µm. Macrophages were stained by targeting the F4/80 receptor expressed on mouse macrophages using immunohistochemistry with specific antibodies. Brown color indicates positively stained macrophages. Magnification 400-fold. Bar = 100 µm. (B) Positively stained macrophages were enumerated along a stretch of 50 µm of lamina muscularis for both lamina propria and sub-mucosa (see materials and methods). SIHUMI mice colonized with both A. muciniphila and S. Typhimurium had the highest macrophage infiltration scores compared to the other groups (see Figure. 1). Data are expressed as median with range. n = 5 mice per group. *P<0.05, **P<0.01, ***P<0.001. Amuc: A. muciniphila; S. Tm: S. Typhimurium.
Figure 7. SIHUMI mice with both A. muciniphila and S. Typhimurium display increased MUC2 mRNA levels (A) and reduced numbers of mucin filled goblet cells (B and C).
(A) mRNA was extracted from cecum mucosa of mice belonging to either one of four groups: SIHUMI, SIHUMI-A, SIHUMI-S and SIHUMI-AS. MUC2 mRNA from cecum mucosa was converted to cDNA and expression levels were quantified using real-time PCR (see materials and methods). SIHUMI-A and SIHUMI-AS mice showed significantly higher MUC2 gene expression compared to the other two groups, harboring no A. muciniphila. Data are expressed as mean±standard error. n = 6 per group. *P<0.05, **P<0.01, ***P<0.001. Amuc: A. muciniphila; S. Tm: S. Typhimurium. (B) Formalin fixed cecal tissue sections from SIHUMI, SIHUMI-A, SIHUMI-S and SIHUMI-AS mice were stained with alcian blue (pH-2.5) and haematoxylin. Images are representative of 5 mice per group. Magnification 400-fold. SIHUMI-AS mice display the lowest number of positively stained mucin-filled goblet cells compared to the other three groups. The bar represents 100 µm. (C) Quantitative analysis of the number of acidic mucin-filled goblet cells (blue) enumerated in cecal tissue sections from SIHUMI, SIHUMI-A, SIHUMI-S and SIHUMI-AS mice for a 50 µm stretch of lamina muscularis corresponding to approximately 30 cecal crypts per section. Two sections per mouse were analyzed. The number of cecal mucin filled goblet cells was elevated when A. muciniphila was present (SIHUMI-A) but the concomitant presence of S. Typhimurium (SIHUMI-AS) resulted in the lowest number of mucin filled goblet cells of gnotobiotic SIHUMI mice compared to the other mouse groups. Data are expressed as mean±standard error. n = 5 mice. *P<0.05, **P<0.01, ***P<0.001. Amuc: A. muciniphila; S. Tm: S. Typhimurium.
Figure 8. SIHUMI mice colonized with both A. muciniphila and S. Typhimurium display reduced mucus sulphation.
Formalin fixed thin sections (4 µm) of cecal tissue of mice belonging to either one of four groups: SIHUMI, SIHUMI-A, SIHUMI-S and SIHUMI-AS (see Figure. 1) were stained with high iron diamine (HID)/AB at pH-2.5 and subsequently analyzed. Brown color indicates sulphated mucins while blue color indicates sialylated mucins. SIHUMI-AS mice display few sulphated mucins compared to the other mouse groups. Magnification 400×. Bars indicate 100 µm.
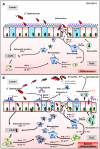
Figure 9. Hypothetical Scheme.
The presence of A. muciniphila, leads to the exacerbation of S. Typhimurium-induced intestinal inflammation. We propose that the presence of A. muciniphila causes changes in mucin composition and production, which in turn facilitates the invasion of S. Typhimurium into the host. Increased inflammatory status was characterized by increased pro-inflammatory cytokines, increased macrophage infiltration and invasion of the pathogen into the lymph nodes, reduced number of mucin-filled goblet cells in SIHUMI-AS mice (B) compared to SIHUMI-S mice (A). Our data suggests that in the presence of both A. muciniphila and S. Typhimurium, mucus sulphation is diminished and this may facilitate the access of S. Typhimurium to sialic acid in mucus. Sialic acid may serve as a substrate and adhesion site for S. Typhimurium in the gut , . Increased gene expression of IFN-γ and IP-10 indicate an increased NK-cell recruitment. mLN - mesenteric lymph nodes, NK- Natural killer cells. (↑ increased; ↓ decreased; grey dotted line: assumed processes including lectin-sialic acid binding , M-cells for pathogen transit , , ; black line: supported by data of the present study).
Similar articles
- Akkermansia muciniphila strain ATCC BAA-835 does not promote short-term intestinal inflammation in gnotobiotic interleukin-10-deficient mice.
Ring C, Klopfleisch R, Dahlke K, Basic M, Bleich A, Blaut M. Ring C, et al. Gut Microbes. 2019;10(2):188-203. doi: 10.1080/19490976.2018.1511663. Epub 2018 Sep 25. Gut Microbes. 2019. PMID: 30252588 Free PMC article. - Live and pasteurized Akkermansia muciniphila decrease susceptibility to Salmonella Typhimurium infection in mice.
Liu J, Liu H, Liu H, Teng Y, Qin N, Ren X, Xia X. Liu J, et al. J Adv Res. 2023 Oct;52:89-102. doi: 10.1016/j.jare.2023.03.008. Epub 2023 Mar 28. J Adv Res. 2023. PMID: 36996967 Free PMC article. - Strain-Specific Anti-inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice.
Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Zhai R, et al. Front Cell Infect Microbiol. 2019 Jul 5;9:239. doi: 10.3389/fcimb.2019.00239. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31334133 Free PMC article. - Akkermansia muciniphila and Alcohol-Related Liver Diseases. A Systematic Review.
Sparfel L, Ratodiarivony S, Boutet-Robinet E, Ellero-Simatos S, Jolivet-Gougeon A. Sparfel L, et al. Mol Nutr Food Res. 2024 Jan;68(2):e2300510. doi: 10.1002/mnfr.202300510. Epub 2023 Dec 7. Mol Nutr Food Res. 2024. PMID: 38059838 Review. - Akkermansia muciniphila and its role in regulating host functions.
Derrien M, Belzer C, de Vos WM. Derrien M, et al. Microb Pathog. 2017 May;106:171-181. doi: 10.1016/j.micpath.2016.02.005. Epub 2016 Feb 11. Microb Pathog. 2017. PMID: 26875998 Review.
Cited by
- Beneficial Effects of Natural Mineral Waters on Intestinal Inflammation and the Mucosa-Associated Microbiota.
Barnich N, Rodrigues M, Sauvanet P, Chevarin C, Denis S, Le Goff O, Faure-Imbert D, Hanh T, Roques CF, Chassaing B, Alric M. Barnich N, et al. Int J Mol Sci. 2021 Apr 21;22(9):4336. doi: 10.3390/ijms22094336. Int J Mol Sci. 2021. PMID: 33919372 Free PMC article. - Aging and serum MCP-1 are associated with gut microbiome composition in a murine model.
Conley MN, Wong CP, Duyck KM, Hord N, Ho E, Sharpton TJ. Conley MN, et al. PeerJ. 2016 Mar 31;4:e1854. doi: 10.7717/peerj.1854. eCollection 2016. PeerJ. 2016. PMID: 27069796 Free PMC article. - Direct impact of commonly used dietary emulsifiers on human gut microbiota.
Naimi S, Viennois E, Gewirtz AT, Chassaing B. Naimi S, et al. Microbiome. 2021 Mar 22;9(1):66. doi: 10.1186/s40168-020-00996-6. Microbiome. 2021. PMID: 33752754 Free PMC article. - Daesiho-Tang Is an Effective Herbal Formulation in Attenuation of Obesity in Mice through Alteration of Gene Expression and Modulation of Intestinal Microbiota.
Hussain A, Yadav MK, Bose S, Wang JH, Lim D, Song YK, Ko SG, Kim H. Hussain A, et al. PLoS One. 2016 Nov 3;11(11):e0165483. doi: 10.1371/journal.pone.0165483. eCollection 2016. PLoS One. 2016. PMID: 27812119 Free PMC article. - Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice.
Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, Ye J, Fang D, Wu J, Jiang X, Shi D, Li L. Bian X, et al. Front Microbiol. 2019 Oct 1;10:2259. doi: 10.3389/fmicb.2019.02259. eCollection 2019. Front Microbiol. 2019. PMID: 31632373 Free PMC article.
References
- Sartor RB (2009) Microbial-host interactions in inflammatory bowel diseases and experimental colitis. Nestle Nutr Workshop Ser Pediatr Program 64: 121–132; discussion 132–127, 251–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work has been funded by the Deutsche Forschungsgemeinschaft (wwww.dfg.de), Collaborative Research Center 852. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials