Glia maturation factor deficiency suppresses 1-methyl-4-phenylpyridinium-induced oxidative stress in astrocytes - PubMed (original) (raw)
Glia maturation factor deficiency suppresses 1-methyl-4-phenylpyridinium-induced oxidative stress in astrocytes
Mohammad Moshahid Khan et al. J Mol Neurosci. 2014 Aug.
Abstract
Inflammation is closely intertwined with pathogenesis of Parkinson's disease (PD). Increasing evidence suggests that inhibition of glia-mediated inflammation might represent a promising therapeutic target for PD. Glia maturation factor (GMF), an inflammatory protein, predominantly localized in astrocytes is previously isolated, sequenced and cloned in our laboratory. In the present investigation, we demonstrate that GMF-deficiency in astrocytes upregulates the antioxidant status and limit the extent of lipid peroxidation and production of reactive oxygen species (ROS) along with diminished nuclear factor-κB-mediated inflammatory responses in 1-methyl-4-phenylpyridinium (MPP(+))-induced toxicity. Primary astrocytes obtained from wild-type (Wt) and GMF-deficient (GMF-KO) mice were treated with 5, 10, and 20 μM MPP(+) for 24, 48, and 72 h in vitro. Our results show decreased release of ROS and increased level of glutathione in astrocytes obtained from GMF-KO mice when compared to astrocytes derived from Wt mice following MPP(+) treatment. Additionally, we found decreased activity of NF-κB, and reduced levels of proinflammatory tumor necrosis factor- α, interleukin-1β (IL-1β), IL-17, IL-33, and chemokine (C-C motif) ligand 2 (CCL2) in GMF-KO astrocytes when compared to Wt astrocytes. Our overall results suggest that GMF-KO astrocytes are significantly resistant to MPP(+) toxicity when compared to Wt astrocytes.
Figures
Fig. 1
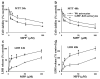
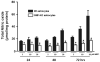
Primary cultures of astrocytes derived from Wt and GMF-KO mice were incubated with various doses of MPP+ (0–100 μM) for 24 and 48 h. MPP+-induced cytotoxicity was measured by MTT reduction and LDH release assays. a MTT assay show higher cell viability in GMF-KO astrocytes when compared to Wt astrocytes, and b Wt astrocytes released more LDH when compared to GMF-KO astrocytes following MPP+ treatments. *p<0.05 (_n_=4), compared with the Wt astrocytes and GMF-KO astrocytes treated with MPP+ by student t test analysis
Fig. 2
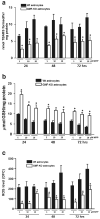
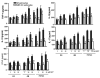
Primary cultures of astrocytes derived from Wt and GMF-KO mice were incubated with MPP+ (5, 10, 20 μM) for 24, 48, and 72 h. Levels TBARS (a), GSH (b), and ROS (c) were assayed in the cell lysate. TBARS and GSH (_n_=4); ROS (_n_=5); Values are means±SEM. *p<0.05, compared with the Wt astrocytes and GMF-KO astrocytes treated with MPP+
Fig. 3
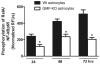
Reduction in NF-κB activity in GMF-KO astrocytes compared to Wt primary mouse astrocytes. Astrocytes were seeded and treated with 20 μM MPP+ for 24, 48, and 72 h and levels of NF-κB activity were measured by ELISA according to manufacturer's protocol. A significant time-dependent suppression of NF-κB activation following MPP+ treatment was seen in GMF-KO astrocytes as compared with Wt astrocytes. Values are means±SEM (_n_=6). *p<0.05, compared with the Wt astrocytes and GMF-KO astrocytes treated with MPP+
Fig. 4
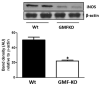
Downregulation of MPP+-dependent proinflammatory iNOS expression in GMF-KO astrocytes. Astrocytes derived from Wt and GMF-KO mice were incubated with MPP+ (20 μM) for 72 h. Cell lysates (35 μg protein per lane) were subjected to SDS-polyacrylamide gel electrophoresis followed by electroblotting. The blots were probed with anti-iNOS antibody and anti-β-actin antibody. Actin served as internal marker showing equal sample loading. Bands intensity was measured by densitometry and quantified using NIH-Image J software. Expression of iNOS was significantly decreased in GMF-KO astrocytes when compared to Wt astrocytes at 20 μM concentration of MPP+ after 72 h. Values are means±SEM (_n_=3). *p<0.05, compared with the Wt astrocytes and GMF-KO astrocytes treated with MPP+, AU arbitrary units
Fig. 5
Nitric oxide (NO) levels were significantly decreased in GMF-KO astrocytes following MPP+ treatment in a dose- (5, 10, and 20 μM) and time-dependent (24, 48, and 72 h) manner. Astrocytes were incubated with MPP+ for 24, 48, and 72 h and then the culture media were collected for NO assay using commercial kit. Values are expressed as means±SEM (_n_=5). *p<0.05, compared with the Wt astrocytes and GMF-KO astrocytes treated with MPP
Fig. 6
Reduced expressions of inflammatory cytokines/chemokine in primary cultures of GMF-KO astrocytes compared to Wt astrocytes following MPP+ treatment. Astrocytes were incubated with MPP+ for 24, 48, and 72 h at 5, 10, and 20 μM MPP+. After the incubation period was over the culture media were collected for the assay of proinflammatory cytokines and chemokine by ELISA. Levels of TNF-α, IL-1β, IL-17, IL-33, and CCL2 were significantly decreased in the GMF-KO astrocytes when compared to the Wt astrocytes in dose- (5, 10, and 20 μM) and time-dependent manner (24, 48, and 72 h) following MPP+ treatment. Values are means±SEM (_n_=5). *p<0.05, compared with the Wt astrocytes and GMF-KO astrocytes treated with MPP+
Similar articles
- Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins α-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators.
Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, Santillan DA, Santillan MK, Zaheer A. Kempuraj D, et al. PLoS One. 2015 Aug 14;10(8):e0135776. doi: 10.1371/journal.pone.0135776. eCollection 2015. PLoS One. 2015. PMID: 26275153 Free PMC article. - Suppression of glia maturation factor expression prevents 1-methyl-4-phenylpyridinium (MPP⁺)-induced loss of mesencephalic dopaminergic neurons.
Khan MM, Zaheer S, Nehman J, Zaheer A. Khan MM, et al. Neuroscience. 2014 Sep 26;277:196-205. doi: 10.1016/j.neuroscience.2014.07.003. Epub 2014 Jul 10. Neuroscience. 2014. PMID: 25016212 Free PMC article. - Glia Maturation Factor and Mast Cell-Dependent Expression of Inflammatory Mediators and Proteinase Activated Receptor-2 in Neuroinflammation.
Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Kumar KK, Yelam A, Kaur H, Dubova I, Raikwar SP, Iyer SS, Zaheer A. Kempuraj D, et al. J Alzheimers Dis. 2018;66(3):1117-1129. doi: 10.3233/JAD-180786. J Alzheimers Dis. 2018. PMID: 30372685 Free PMC article. - Mast Cells Release Chemokine CCL2 in Response to Parkinsonian Toxin 1-Methyl-4-Phenyl-Pyridinium (MPP(+)).
Kempuraj D, Thangavel R, Fattal R, Pattani S, Yang E, Zaheer S, Santillan DA, Santillan MK, Zaheer A. Kempuraj D, et al. Neurochem Res. 2016 May;41(5):1042-9. doi: 10.1007/s11064-015-1790-z. Epub 2015 Dec 8. Neurochem Res. 2016. PMID: 26646004 Free PMC article. - Mast Cell Proteases Activate Astrocytes and Glia-Neurons and Release Interleukin-33 by Activating p38 and ERK1/2 MAPKs and NF-κB.
Kempuraj D, Thangavel R, Selvakumar GP, Ahmed ME, Zaheer S, Raikwar SP, Zahoor H, Saeed D, Dubova I, Giler G, Herr S, Iyer SS, Zaheer A. Kempuraj D, et al. Mol Neurobiol. 2019 Mar;56(3):1681-1693. doi: 10.1007/s12035-018-1177-7. Epub 2018 Jun 18. Mol Neurobiol. 2019. PMID: 29916143 Free PMC article.
Cited by
- Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins α-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators.
Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, Santillan DA, Santillan MK, Zaheer A. Kempuraj D, et al. PLoS One. 2015 Aug 14;10(8):e0135776. doi: 10.1371/journal.pone.0135776. eCollection 2015. PLoS One. 2015. PMID: 26275153 Free PMC article. - The TAK1→IKKβ→TPL2→MKK1/MKK2 Signaling Cascade Regulates IL-33 Expression in Cystic Fibrosis Airway Epithelial Cells Following Infection by Pseudomonas aeruginosa.
Farias R, Rousseau S. Farias R, et al. Front Cell Dev Biol. 2016 Jan 11;3:87. doi: 10.3389/fcell.2015.00087. eCollection 2015. Front Cell Dev Biol. 2016. PMID: 26793709 Free PMC article. - First description of enhanced expression of glia maturation factor-beta in experimental toxoplasmic encephalitis.
Dincel GC. Dincel GC. J Int Med Res. 2017 Dec;45(6):1670-1679. doi: 10.1177/0300060517700320. Epub 2017 Aug 4. J Int Med Res. 2017. PMID: 28774213 Free PMC article. - Absence of glia maturation factor protects dopaminergic neurons and improves motor behavior in mouse model of parkinsonism.
Khan MM, Zaheer S, Thangavel R, Patel M, Kempuraj D, Zaheer A. Khan MM, et al. Neurochem Res. 2015 May;40(5):980-90. doi: 10.1007/s11064-015-1553-x. Epub 2015 Mar 10. Neurochem Res. 2015. PMID: 25754447 Free PMC article. - Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium ion (MPP(+))-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway.
Yu S, Wang X, He X, Wang Y, Gao S, Ren L, Shi Y. Yu S, et al. Cell Stress Chaperones. 2016 Jul;21(4):697-705. doi: 10.1007/s12192-016-0695-3. Epub 2016 May 10. Cell Stress Chaperones. 2016. PMID: 27164829 Free PMC article.
References
- Assouline JG, Bosch EP, Lim R. Purification of rat Schwann cells from cultures of peripheral nerve: an immunoselective method using surfaces coated with anti-immunoglobulin antibodies. Brain Res. 1983;277:389–392. - PubMed
- Browne TC, McQuillan K, McManus RM, O'Reilly JA, Mills KH, Lynch MA. IFN-gamma Production by amyloid beta-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. J Immunol. 2013;190:2241–2251. - PubMed
- Delgado M, Ganea D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: potential therapeutic role in brain trauma. FASEB J. 2003;17:1922–1924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials