The synthetic α-bromo-2',3,4,4'-tetramethoxychalcone (α-Br-TMC) inhibits the JAK/STAT signaling pathway - PubMed (original) (raw)
The synthetic α-bromo-2',3,4,4'-tetramethoxychalcone (α-Br-TMC) inhibits the JAK/STAT signaling pathway
Sophia Pinz et al. PLoS One. 2014.
Erratum in
- PLoS One. 2014;9(8):e105845
Abstract
Signal transducer and activator of transcription STAT5 and its upstream activating kinase JAK2 are essential mediators of cytokine signaling. Their activity is normally tightly regulated and transient. However, constitutive activation of STAT5 is found in numerous cancers and a driving force for malignant transformation. We describe here the identification of the synthetic chalcone α-Br-2',3,4,4'-tetramethoxychalcone (α-Br-TMC) as a novel JAK/STAT inhibitor. Using the non-transformed IL-3-dependent B cell line Ba/F3 and its oncogenic derivative Ba/F3-1*6 expressing constitutively activated STAT5, we show that α-Br-TMC targets the JAK/STAT pathway at multiple levels, inhibiting both JAK2 and STAT5 phosphorylation. Moreover, α-Br-TMC alters the mobility of STAT5A/B proteins in SDS-PAGE, indicating a change in their post-translational modification state. These alterations correlate with a decreased association of STAT5 and RNA polymerase II with STAT5 target genes in chromatin immunoprecipitation assays. Interestingly, expression of STAT5 target genes such as Cis and c-Myc was differentially regulated by α-Br-TMC in normal and cancer cells. While both genes were inhibited in IL-3-stimulated Ba/F3 cells, expression of the oncogene c-Myc was down-regulated and that of the tumor suppressor gene Cis was up-regulated in transformed Ba/F3-1*6 cells. The synthetic chalcone α-Br-TMC might therefore represent a promising novel anticancer agent for therapeutic intervention in STAT5-associated malignancies.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
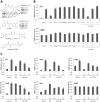
Figure 1. α-Br-TMC inhibits IL-3-mediated induction of STAT5-dependent and -independent genes in Ba/F3 cells.
(A) Structure of the natural and synthetic chalcones used in this study. Second-order rate constants k2 values of compounds obtained using cysteamine in TRIS-HCl buffer pH 7.4: ethylene glycol 20:80 are taken from refs. , . (B) Ba/F3 cells were pre-treated 30 minutes with 0.2 µM (TSA) or 20 µM (all other compounds) candidate inhibitors and stimulated 60 minutes with 5 ng/mL IL-3, as described in Materials and Methods. Following cell harvest, expression of the STAT5 target gene Cis and of the housekeeping gene 36b4 were measured by quantitative RT-PCR, as described in Materials and Methods. Together with TSA, α-Br-TMC was the only compound able to inhibit expression of the STAT5 target gene Cis. (C) Ba/F3 cells were pre-treated 30 minutes with the indicated concentrations of TSA and α-Br-TMC and further stimulated with 5 ng/mL IL-3 for 30 minutes. Expression of STAT5-dependent (Cis, Osm, c-Myc) and -independent (JunB, Ho-1, 36b4) genes was analyzed by quantitative RT-PCR. DMSO (vehicle) was adjusted to 0.02% final concentration in all conditions. Curcu., curcumin.
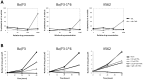
Figure 2. Effect of α-Br-TMC on cytotoxicity and viability of normal (Ba/F3) and transformed (Ba/F3-1*6, K562) cells.
(A) Cells were pre-treated 30 minutes with 0.001, 0.01, 0.1 and 1 µM TSA or with 0.1, 1, 10 and 100 µM α-Br-TMC before starting the WST-1 assay. IL-3 (5 ng/mL) was added to rested Ba/F3 cells at the same time as the WST-1 reagent to mimic the IL-3 stimulation conditions used in other assays. DMSO (vehicle) concentration was adjusted to 0.1% final in all conditions. OD measurement was performed after 90 minutes incubation with the WST-1 reagent, and the percentage of cytotoxicity was normalized to the vehicle control. (B) Growing Ba/F3, Ba/F3-1*6 and K562 cells were incubated for 24 and 48 hours in the presence of the indicated concentrations of TSA and α-Br-TMC. Cell viability was measured by Trypan Blue exclusion assay.
Figure 3. α-Br-TMC inhibits both JAK2 and STAT5 phosphorylation.
Ba/F3 cells were pre-treated 30 minutes with the indicated concentrations of TSA and α-Br-TMC (A) or with 10 µM α-Br-TMC and DMSO vehicle (B, C) and further stimulated with 5 ng/mL IL-3 for 30 minutes (A), 15 minutes (B) or 5 minutes (C). Final DMSO concentration was 0.02% in (A) and 0.01% in (B, C). Protein whole cell extracts (panels A, C) or cytosolic (C) and nuclear (N) extracts (panel B) were prepared as described in Materials and Methods and analyzed by Western-blot using antibodies specific for phospho-STAT5 (pSTAT5), phospho-JAK2 (pJAK2), STAT5A, STAT5B, STAT5A and B, JAK2 and α-tubulin (loading control and cytosolic-specific marker). SDS-PAGE in (C) was shorter, explaining why the STAT5 mobility patterns (α-Br-TMC-induced shift and STAT5A and B doublet) are not as apparent as on immunoblots in (A, B).
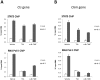
Figure 4. RNA polymerase II recruitment to the Cis and Osm promoters is impeded in Ba/F3 cells treated with α-Br-TMC.
Ba/F3 cells were pre-treated 30 minutes with 0.2 µM TSA or 10 µM α-Br-TMC before being stimulated with 5 ng/mL IL-3 for 30 minutes. DMSO (vehicle) was adjusted to 0.02% in all conditions. Chromatin immunoprecipitation (ChIP) was performed as described in Materials and Methods using antibodies directed against STAT5 or RNA polymerase II (RNA Pol II) proteins. Co-precipitated genomic DNA was analyzed by quantitative PCR using primers specific for the STAT5 binding sites (STAT5 ChIP) or the transcription start site (RNA Pol II ChIP) of the mouse Cis (A) and Osm (B) genes.
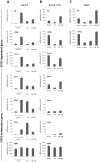
Figure 5. α-Br-TMC exerts distinct effects in normal and cancer cells.
Ba/F3 (A), its caSTAT5-transformed counterpart Ba/F3-1*6 (B) and human leukemic K562 (C) cells were treated 90 minutes with 0.2 µM TSA, 10 µM α-Br-TMC or 1 µM Imatinib. Ba/F3 cells (A) were stimulated with 5 ng/mL IL-3 after an initial 30 minute drug pre-treatment (hence subjected to a 60 minute IL-3 stimulation). DMSO (vehicle) final concentration was adjusted to 0.02% in all conditions. Expression of STAT5-dependent (Cis, Osm, c-Myc, Pim-1) and -independent (JunB, c-Fos, 36b4) genes was analyzed by quantitative RT-PCR. Gene expression data were normalized to mouse ribosomal S9 (A, B) or to human Lamin A/C (LMNA) (C) housekeeping gene-encoded mRNAs. (A, B) Normalized data are presented with adjusted Y-axis scale for a direct comparison of mRNA levels in the respective normal and transformed Ba/F3 and Ba/F3-1*6 cell lines.
Figure 6. α-Br-TMC inhibits the STAT5 signaling pathway in both a JAK2-dependent and -independent manner.
(A) Ba/F3-1*6 and K562 cells were treated for 60 minutes with the indicated compounds. Protein whole cell extracts were analyzed by Western-Blot using antibodies specific for pSTAT5, STAT5A, STAT5B, STAT5A and B and α-tubulin as a loading control. (B) Ba/F3 cells were pre-treated 30 minutes with 10 µM α-Br-TMC and stimulated with 5 ng/mL IL-3 for 5 minutes. Ba/F3-1*6 and K562 cells were treated with 10 µM α-Br-TMC for 90 minutes. DMSO (Veh.) was adjusted to 0.01% in all conditions. Protein whole cell extracts were analyzed by Western-Blot using antibodies specific for pSTAT5, pJAK2, STAT5A, STAT5B, STAT5A and B, JAK2 and α-tubulin (loading control).
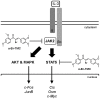
Figure 7. Model of inhibition of the JAK2/STAT5 pathway by α-Br-TMC.
IL-3 binding to the α/βc chains of the IL-3 receptor leads to activation of the receptor-associated JAK2 tyrosine kinase by trans-phosphorylation. In turn, JAK2-mediated activation of the STAT5, MAPK and AKT pathways via phosphorylation (broad arrows) results in induced transcription of downstream target genes (thin arrows). We showed that α-Br-TMC inhibits JAK2 phosphorylation, hence impairing JAK2-regulated signaling pathways. In addition and independently of JAK2, α-Br-TMC inhibits STAT5 activity. Concomitantly, STAT5A and STAT5B protein mobility in SDS-PAGE is altered, indicating a change in their post-translational modification state induced by α-Br-TMC, which might be associated to STAT5 altered transcriptional activity.
References
- Grimley PM, Dong F, Rui H (1999) Stat5a and Stat5b: fraternal twins of signal transduction and transcriptional activation. Cytokine Growth Factor Rev 10: 131–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by the Deutsche Forschungsgemeinschaft (Grant No. RA 2010/2-1 to AR), the Deutsche Krebshilfe (Grant No. 109750 to AR), institutional research funds (Foerderlinie C to AR), the Fonds der Chemischen Industrie (Liebig scholarship to SA) and the DAAD (Doctoral scholarship to NA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous