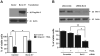Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells - PubMed (original) (raw)
Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells
Dae-Hee Lee et al. Toxicol Appl Pharmacol. 2014.
Abstract
Glioblastoma multiforme (GBM) is the most lethal and aggressive astrocytoma of primary brain tumors in adults. Although there are many clinical trials to induce the cell death of glioblastoma cells, most glioblastoma cells have been reported to be resistant to TRAIL-induced apoptosis. Here, we showed that gingerol as a major component of ginger can induce TRAIL-mediated apoptosis of glioblastoma. Gingerol increased death receptor (DR) 5 levels in a p53-dependent manner. Furthermore, gingerol decreased the expression level of anti-apoptotic proteins (survivin, c-FLIP, Bcl-2, and XIAP) and increased pro-apoptotic protein, Bax and truncate Bid, by generating reactive oxygen species (ROS). We also found that the sensitizing effects of gingerol in TRAIL-induced cell death were blocked by scavenging ROS or overexpressing anti-apoptotic protein (Bcl-2). Therefore, we showed the functions of gingerol as a sensitizing agent to induce cell death of TRAIL-resistant glioblastoma cells. This study gives rise to the possibility of applying gingerol as an anti-tumor agent that can be used for the purpose of combination treatment with TRAIL in TRAIL-resistant glioblastoma tumor therapy.
Keywords: Apoptosis; Gingerol; Glioblastoma; Reactive oxygen species (ROS); Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL); p53.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement: The authors declare that they have no competing interests.
Figures
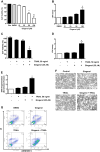
Fig. 1
Sensitizing effect of gingerol in TRAIL-induced apoptosis of U87 glioblastoma cells. (A and B) Cells were treated with DMSO (sham control) or various concentrations (10–100μM) of gingerol for 24 h. (C, D, and E) Cells were incubated in the presence or absence of TRAIL (50 ng/ml) and/or gingerol (25 μM) for 24 h. (F) Microscopic cell morphologies. Scale bar: 100 μm. (G) The cells were stained with annexin V and propidium iodide (PI), followed by FACS analysis. (H) The cell lysates were analyzed by western blotting using indicated antibodies. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. Asterisk * represents statistically significantly difference between control and gingerol-treated cells at p < 0.05.
Fig. 1
Sensitizing effect of gingerol in TRAIL-induced apoptosis of U87 glioblastoma cells. (A and B) Cells were treated with DMSO (sham control) or various concentrations (10–100μM) of gingerol for 24 h. (C, D, and E) Cells were incubated in the presence or absence of TRAIL (50 ng/ml) and/or gingerol (25 μM) for 24 h. (F) Microscopic cell morphologies. Scale bar: 100 μm. (G) The cells were stained with annexin V and propidium iodide (PI), followed by FACS analysis. (H) The cell lysates were analyzed by western blotting using indicated antibodies. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. Asterisk * represents statistically significantly difference between control and gingerol-treated cells at p < 0.05.
Fig. 2
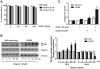
Gingerol can regulate the expression of death receptors (DR) 5, but not DR4. (A) U87 cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h). Western blotting analysis was performed with DR4 and DR5 antibodies. (B) U87 cells were incubated with DMSO or gingerol (25 μM) for 24 h and stained with DR4 and DR5 antibodies, followed by FACS analysis (left panels) and immunocytochemistry (right panels). Scale bar: 100 μm. (C) The indicated cell types were incubated with DMSO or gingerol (25 μM) for 24 h. The cell lysates were analyzed by western blotting using DR4 and DR5 antibodies. (D) U87 cells were transfected with Myc-DR5 plasmid or pcDNA (control). After treatment with TRAIL for 4 h, western blot analysis demonstrated that Myc-DR5-induced DR5 overexpression increased apoptosis compared with pcDNA. (E) U87 cells were incubated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h) and then analyzed by western blotting using p53 antibody. (F) HCT116 (p53+/+) and HCT116 (p53−/−) cells were incubated with indicated gingerol (0, 10, 25, 50 μM) for 24 h. The cell lysates were analyzed by western blotting using p53, DR4, and DR5 antibodies. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. *p < 0.05 vs control. (G) Cell viability was determined using the trypan blue dye exclusion assay. Error bars represent the mean ± SE from six separate experiments. *Significant difference between TRAIL and TRAIL + gingerol-treated cells at p < 0.05. These results are representative of data obtained from at least five independent experiments.
Fig. 2
Gingerol can regulate the expression of death receptors (DR) 5, but not DR4. (A) U87 cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h). Western blotting analysis was performed with DR4 and DR5 antibodies. (B) U87 cells were incubated with DMSO or gingerol (25 μM) for 24 h and stained with DR4 and DR5 antibodies, followed by FACS analysis (left panels) and immunocytochemistry (right panels). Scale bar: 100 μm. (C) The indicated cell types were incubated with DMSO or gingerol (25 μM) for 24 h. The cell lysates were analyzed by western blotting using DR4 and DR5 antibodies. (D) U87 cells were transfected with Myc-DR5 plasmid or pcDNA (control). After treatment with TRAIL for 4 h, western blot analysis demonstrated that Myc-DR5-induced DR5 overexpression increased apoptosis compared with pcDNA. (E) U87 cells were incubated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h) and then analyzed by western blotting using p53 antibody. (F) HCT116 (p53+/+) and HCT116 (p53−/−) cells were incubated with indicated gingerol (0, 10, 25, 50 μM) for 24 h. The cell lysates were analyzed by western blotting using p53, DR4, and DR5 antibodies. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. *p < 0.05 vs control. (G) Cell viability was determined using the trypan blue dye exclusion assay. Error bars represent the mean ± SE from six separate experiments. *Significant difference between TRAIL and TRAIL + gingerol-treated cells at p < 0.05. These results are representative of data obtained from at least five independent experiments.
Fig. 2
Gingerol can regulate the expression of death receptors (DR) 5, but not DR4. (A) U87 cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h). Western blotting analysis was performed with DR4 and DR5 antibodies. (B) U87 cells were incubated with DMSO or gingerol (25 μM) for 24 h and stained with DR4 and DR5 antibodies, followed by FACS analysis (left panels) and immunocytochemistry (right panels). Scale bar: 100 μm. (C) The indicated cell types were incubated with DMSO or gingerol (25 μM) for 24 h. The cell lysates were analyzed by western blotting using DR4 and DR5 antibodies. (D) U87 cells were transfected with Myc-DR5 plasmid or pcDNA (control). After treatment with TRAIL for 4 h, western blot analysis demonstrated that Myc-DR5-induced DR5 overexpression increased apoptosis compared with pcDNA. (E) U87 cells were incubated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h) and then analyzed by western blotting using p53 antibody. (F) HCT116 (p53+/+) and HCT116 (p53−/−) cells were incubated with indicated gingerol (0, 10, 25, 50 μM) for 24 h. The cell lysates were analyzed by western blotting using p53, DR4, and DR5 antibodies. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. *p < 0.05 vs control. (G) Cell viability was determined using the trypan blue dye exclusion assay. Error bars represent the mean ± SE from six separate experiments. *Significant difference between TRAIL and TRAIL + gingerol-treated cells at p < 0.05. These results are representative of data obtained from at least five independent experiments.
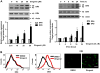
Fig. 3
Modulation of gingerol in the pro-apoptotic and anti-apoptotic signaling pathways in glioblastoma cells. U87 cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h. (A) Western blotting analysis was done by using indicated antibodies (XIAP, Survivin, c-FLIP, Bax, Bcl-2, and Bid). (B) Phosphorylation of MAP kinases was analyzed by western blotting using phosphorylation-specific antibodies (p-ERK, p-p38, and p-JNK). (C) U343 and T98G cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h, followed by western blotting using Bcl-2 antibody. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. *p < 0.05 vs control. These results are representative of data obtained from at least five independent experiments.
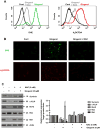
Fig. 4
Gingerol can regulate the pro-apoptotic signaling of TRAIL through ROS generation. U87 cells were treated with gingerol (25 μM) in the presence or absence of N-acetylcysteine (Lluis et al., 2010) for 24 h, and then incubated with dihydroethidium (DHE) or 2′,7′-dichlorofluorescein (H2DCF), followed by (A) FACS analysis and (B) immunocytochemical analysis. Scale bar: 100 μm. (C) The cells were incubated with indicated drugs (25 μM gingerol and 5 mM NAC) for 24 h, and then western blotting was performed by using the indicated antibodies (Survivin, c-FLIP, BAX, Bcl-2, and Bid). (D) The cells were treated with indicated reagents (TRAIL, gingerol and NAC) for 24 h. The cell lysates were analyzed by western blotting using indicated antibodies (caspase-3,-8 and PARP-1). Data is presented as arbitrary values and results are expressed as the mean ±SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. *p < 0.05 vs control. These results are representative of data obtained from at least five independent experiments.
Fig. 4
Gingerol can regulate the pro-apoptotic signaling of TRAIL through ROS generation. U87 cells were treated with gingerol (25 μM) in the presence or absence of N-acetylcysteine (Lluis et al., 2010) for 24 h, and then incubated with dihydroethidium (DHE) or 2′,7′-dichlorofluorescein (H2DCF), followed by (A) FACS analysis and (B) immunocytochemical analysis. Scale bar: 100 μm. (C) The cells were incubated with indicated drugs (25 μM gingerol and 5 mM NAC) for 24 h, and then western blotting was performed by using the indicated antibodies (Survivin, c-FLIP, BAX, Bcl-2, and Bid). (D) The cells were treated with indicated reagents (TRAIL, gingerol and NAC) for 24 h. The cell lysates were analyzed by western blotting using indicated antibodies (caspase-3,-8 and PARP-1). Data is presented as arbitrary values and results are expressed as the mean ±SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. *p < 0.05 vs control. These results are representative of data obtained from at least five independent experiments.
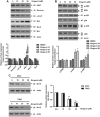
Fig. 5
Effect of Bcl-2 in the sensitizing function of gingerol. (A) Vector or Bcl-2-overexpressing U87 stable cell lines (upper panel) were treated with TRAIL and gingerol for 24 h. The cell lysates were analyzed by western blotting of Flag antibody. Actin was used to confirm the equal amount of proteins loaded in each lane. All results are representative of the data obtained by five independent experiments. The cells were then analyzed by MTT assay. (B) Bcl-2 was silenced by si-RNA in U87 cells (upper panel). The cells were then treated with TRAIL for 24 h, followed by MTT analysis. Results shown are representative of six independent experiments.
Fig. 6
Astrocytes are resistant to the combined treatment with gingerol and TRAIL. (A) Human astrocytes were treated with or without gingerol for 30 min and further treated with TRAIL for 16 h at the indicated concentrations. Cellular viability was assessed using trypan blue exclusion assay. (B) Following treatment of astrocytes or U87MG cells with 25 μM gingerol for the indicated times, western blotting of DR5 and Bcl-2 antibodies. Actin was used to confirm the equal amount of proteins loaded in each lane. (C) Astrocytes or U87MG cells were treated with 25 μM gingerol alone, 100 ng/ml TRAIL alone or a combination with gingerol and TRAIL for 16 h. Caspase-3/7 activity is shown. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired _t_-test. *p < 0.05 vs control. Results shown are representative of six independent experiments.
Fig. 7
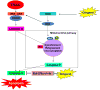
Schematic diagram of working model of gingerol for sensitizing TRAIL-induced apoptosis.
Similar articles
- Upregulation of Death Receptor 5 and Production of Reactive Oxygen Species Mediate Sensitization of PC-3 Prostate Cancer Cells to TRAIL Induced Apoptosis by Vitisin A.
Shin D, Kwon HY, Sohn EJ, Nam MS, Kim JH, Lee JC, Ryu SY, Park B, Kim SH. Shin D, et al. Cell Physiol Biochem. 2015;36(3):1151-62. doi: 10.1159/000430286. Epub 2015 Jun 25. Cell Physiol Biochem. 2015. PMID: 26111475 - Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors.
Sung B, Prasad S, Ravindran J, Yadav VR, Aggarwal BB. Sung B, et al. Free Radic Biol Med. 2012 Nov 15;53(10):1977-87. doi: 10.1016/j.freeradbiomed.2012.08.012. Epub 2012 Aug 15. Free Radic Biol Med. 2012. PMID: 22922338 Free PMC article. - Inhibition of the autophagy flux by gingerol enhances TRAIL-induced tumor cell death.
Nazim UM, Jeong JK, Seol JW, Hur J, Eo SK, Lee JH, Park SY. Nazim UM, et al. Oncol Rep. 2015 May;33(5):2331-6. doi: 10.3892/or.2015.3869. Epub 2015 Mar 20. Oncol Rep. 2015. PMID: 25813697 - Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.
Hassanzadeh A, Farshdousti Hagh M, Alivand MR, Akbari AAM, Shams Asenjan K, Saraei R, Solali S. Hassanzadeh A, et al. J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review. - Current approaches in enhancing TRAIL therapies in glioblastoma.
Thang M, Mellows C, Mercer-Smith A, Nguyen P, Hingtgen S. Thang M, et al. Neurooncol Adv. 2023 Apr 21;5(1):vdad047. doi: 10.1093/noajnl/vdad047. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 37215952 Free PMC article. Review.
Cited by
- A Potential Anti-cancer Compound Separated from the Chloroform Extract of the Chinese Medicine Formula Shenqi San.
Shi L, Tu YJ, Ye SQ, Xia Y, Ma CZ, Peng XZ, Liu YW, Ai ZZ, You PT. Shi L, et al. Curr Med Sci. 2020 Feb;40(1):138-144. doi: 10.1007/s11596-020-2157-5. Epub 2020 Mar 13. Curr Med Sci. 2020. PMID: 32166676 - Anticancer Effect of Ginger Extract against Pancreatic Cancer Cells Mainly through Reactive Oxygen Species-Mediated Autotic Cell Death.
Akimoto M, Iizuka M, Kanematsu R, Yoshida M, Takenaga K. Akimoto M, et al. PLoS One. 2015 May 11;10(5):e0126605. doi: 10.1371/journal.pone.0126605. eCollection 2015. PLoS One. 2015. PMID: 25961833 Free PMC article. - Gingerol acts as a potent radiosensitizer in head and neck squamous cell carcinoma.
Rutihinda C, Haroun R, Ordonez JP, Mohssine S, Oweida H, Sharma M, Fares M, Ruiz-Dominguez N, Pacheco MFM, Naasri S, Saidi NE, Oweida AJ. Rutihinda C, et al. Discov Oncol. 2024 Oct 13;15(1):553. doi: 10.1007/s12672-024-01425-y. Discov Oncol. 2024. PMID: 39397185 Free PMC article. - Effect of extracts from eggs of Helix aspersa maxima and Helix aspersa aspersa snails on Caco-2 colon cancer cells.
Matusiewicz M, Marczak K, Kwiecińska B, Kupis J, Zglińska K, Niemiec T, Kosieradzka I. Matusiewicz M, et al. PeerJ. 2022 Apr 12;10:e13217. doi: 10.7717/peerj.13217. eCollection 2022. PeerJ. 2022. PMID: 35433131 Free PMC article. - Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications.
Cosme P, Rodríguez AB, Espino J, Garrido M. Cosme P, et al. Antioxidants (Basel). 2020 Dec 12;9(12):1263. doi: 10.3390/antiox9121263. Antioxidants (Basel). 2020. PMID: 33322700 Free PMC article. Review.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
- Bode AM, Ma WY, Surh YJ, Dong Z. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61:850–853. - PubMed
- Boerman RH, Anderl K, Herath J, Borell T, Johnson N, Schaeffer-Klein J, Kirchhof A, Raap AK, Scheithauer BW, Jenkins RB. The glial and mesenchymal elements of gliosarcomas share similar genetic alterations. J Neuropathol Exp Neurol. 1996;55:973–981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous