ZnT4 (SLC30A4)-null ("lethal milk") mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation - PubMed (original) (raw)
ZnT4 (SLC30A4)-null ("lethal milk") mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation
Nicholas H McCormick et al. Am J Physiol Regul Integr Comp Physiol. 2016.
Abstract
During lactation, highly specialized secretory mammary epithelial cells (MECs) produce and secrete huge quantities of nutrients and nonnutritive factors into breast milk. The zinc (Zn) transporter ZnT4 (SLC30A4) transports Zn into the trans-Golgi apparatus for lactose synthesis, and across the apical cell membrane for efflux from MECs into milk. This is consistent with observations in "lethal milk" (lm/lm) mice, which have a truncation mutation in SLC30A4, and present with not only low milk Zn concentration, but also smaller mammary glands, decreased milk volume, and lactation failure by lactation day 2. However, the molecular underpinnings of these defects are not understood. Here, we used lactating C57BL/6J(lm/lm) (ZnT4-null) mice to explore the consequences of a ZnT4-null phenotype on mammary gland function during early lactation. Lactating C57BL/6J(lm/lm) mice had significantly fewer, smaller, and collapsed alveoli comprising swollen, lipid-filled MECs during early lactation. These defects were associated with decreased Akt expression and STAT5 activation, indicative of defects in MEC secretion. In addition, increased expression of ZnT2, TNF-α, and cleaved e-cadherin concomitant with increased activation of STAT3 implicated the loss of ZnT4 in precocious activation of involution. Collectively, our study indicates that the loss of ZnT4 has profound consequences on MEC secretion and may promote tissue remodeling in the mammary gland during early lactation.
Keywords: SLC30A4; ZnT4; lactation; mammary gland; zinc.
Copyright © 2016 the American Physiological Society.
Figures
Fig. 1.
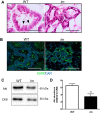
A ZnT4-null phenotype in C57BL/6J_lm/lm_ mice leads to reduced secretory epithelium. A: representative immunoblot of ZnT4 in total membrane fractions prepared from wild-type (WT) and C57BL/6J_lm/lm_ (lm) mammary glands. β-actin was used as a loading control. B: representative whole mount images of mammary glands from WT and lm mice on lactation day 2. Magnification: ×0.5; scale bar = 0.5 mm. C: hematoxylin and eosin (H&E)-stained images of mammary glands from WT and lm mice on lactation day 2 at ×4 magnification (i, ii: scale bar: 250 μm) and ×10 magnification (iii, iv: scale bar = 100 μm). D: data represent mean alveolar diameter (μm) ± SD, n = 4 mice/genotype. *Significant effect of genotype, P < 0.05. E: representative immunoblots of the luminal epithelial cell marker, cytokeratin 8 (CK8) in cell lysates from WT and lm mammary glands. β-actin was used as a loading control. F: data represent mean CK8 protein abundance normalized to β-actin ± SD; n = 3 mice/genotype. *Significant effect of genotype, P < 0.05. G: representative immunoblots of phospho-STAT5 (p-STAT5) and total STAT5 in cell lysates from mammary glands of WT and lm mice. CK8 was used as a loading control. H: quantification of STAT5 activation. Data represent mean ratio of p-STAT5/total STAT5 normalized to CK8 ± SD; n = 3 mice/genotype. **Significant effect of genotype, P < 0.01.
Fig. 2.
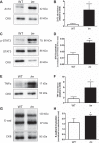
Secretory defects in C57BL/6J_lm/lm_ mammary glands. A: H&E-stained images of mammary glands from WT and C57BL/6J_lm/lm_ (lm) mice on lactation day 2 at ×100 magnification; scale bar = 100 μm. Black arrowheads mark active fusion of lipid droplets. B: representative images of adipophilin (ADRP; green) in mammary glands from WT and lm mice on lactation day 2. Nuclei were counterstained with DAPI (blue). Magnification: ×63; scale bar = 100 μm. C: representative immunoblots of Akt in cell lysates from WT and lm mammary glands. Cytokeratin 8 (CK8) was used for normalization. D: data represent mean Akt protein abundance relative to CK8 ± SD; n = 3 mice/genotype. **Significant effect of genotype, P < 0.01.
Fig. 3.
ZnT2 abundance is increased in the C57BL/6J_lm/lm_ mammary gland. A: representative immunoblot of ZnT2 in total membrane fractions from mammary glands of C57BL/6J_lm/lm_ (lm) mice and their WT littermates. Cytokeratin 8 (CK8) was used for normalization. B: data represent mean ZnT2 protein abundance relative to cytokeratin 8 (CK8) ± SD; n = 3 mice/genotype from two different experiments. *Significant effect of genotype, P < 0.05. C: representative immunoblots of phospho-STAT3 (p-STAT3) and total STAT3 in cell lysates from mammary glands of WT and lm mice. CK8 was used for normalization. D: quantification of STAT3 activation. Data represent mean ratio of p-STAT3/total STAT3 normalized to CK8 ± SD, n = 3 mice/genotype. **Significant effect of genotype, P < 0.01. E: representative immunoblots of TNF-α in cell lysates from mammary glands of WT and lm mice. CK8 was used as a loading control. F: data represent mean TNF-α protein abundance relative to CK8 ± SD; n = 3 mice/genotype. *Significant effect of genotype, P < 0.05. G: representative immunoblots of mature (120 kDa) and truncated (97 kDa) e-cadherin (e-cad) in cell lysates from mammary glands of WT and lm mice. CK8 was used for normalization. H: E-cadherin truncation. Data represent mean ratio of truncated/mature e-cad normalized to CK8 ± SD; n = 3 mice/genotype. *Significant effect of genotype, P < 0.05.
Similar articles
- ZnT4 provides zinc to zinc-dependent proteins in the trans-Golgi network critical for cell function and Zn export in mammary epithelial cells.
McCormick NH, Kelleher SL. McCormick NH, et al. Am J Physiol Cell Physiol. 2012 Aug 1;303(3):C291-7. doi: 10.1152/ajpcell.00443.2011. Epub 2012 May 23. Am J Physiol Cell Physiol. 2012. PMID: 22621784 Free PMC article. - ZnT2 is critical for lysosome acidification and biogenesis during mammary gland involution.
Rivera OC, Hennigar SR, Kelleher SL. Rivera OC, et al. Am J Physiol Regul Integr Comp Physiol. 2018 Aug 1;315(2):R323-R335. doi: 10.1152/ajpregu.00444.2017. Epub 2018 May 2. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29718697 Free PMC article. - Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation.
Lee S, Hennigar SR, Alam S, Nishida K, Kelleher SL. Lee S, et al. J Biol Chem. 2015 May 22;290(21):13064-78. doi: 10.1074/jbc.M115.637439. Epub 2015 Apr 7. J Biol Chem. 2015. PMID: 25851903 Free PMC article. - The role of Stat3 in apoptosis and mammary gland involution. Conditional deletion of Stat3.
Chapman RS, Lourenco P, Tonner E, Flint D, Selbert S, Takeda K, Akira S, Clarke AR, Watson CJ. Chapman RS, et al. Adv Exp Med Biol. 2000;480:129-38. doi: 10.1007/0-306-46832-8_16. Adv Exp Med Biol. 2000. PMID: 10959419 Review. - Molecular mechanism of mammary gland involution: An update.
Jena MK, Jaswal S, Kumar S, Mohanty AK. Jena MK, et al. Dev Biol. 2019 Jan 15;445(2):145-155. doi: 10.1016/j.ydbio.2018.11.002. Epub 2018 Nov 15. Dev Biol. 2019. PMID: 30448440 Review.
Cited by
- Zinc Signaling in the Mammary Gland: For Better and for Worse.
Chakraborty M, Hershfinkel M. Chakraborty M, et al. Biomedicines. 2021 Sep 12;9(9):1204. doi: 10.3390/biomedicines9091204. Biomedicines. 2021. PMID: 34572390 Free PMC article. Review. - Zinc in Cellular Regulation: The Nature and Significance of "Zinc Signals".
Maret W. Maret W. Int J Mol Sci. 2017 Oct 31;18(11):2285. doi: 10.3390/ijms18112285. Int J Mol Sci. 2017. PMID: 29088067 Free PMC article. Review. - The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective.
Kimura T, Kambe T. Kimura T, et al. Int J Mol Sci. 2016 Mar 4;17(3):336. doi: 10.3390/ijms17030336. Int J Mol Sci. 2016. PMID: 26959009 Free PMC article. Review. - Proteomic Analysis of Zn Depletion/Repletion in the Hormone-Secreting Thyroid Follicular Cell Line FRTL-5.
Guantario B, Capolupo A, Monti MC, Leoni G, Ranaldi G, Tosco A, Marzullo L, Murgia C, Perozzi G. Guantario B, et al. Nutrients. 2018 Dec 14;10(12):1981. doi: 10.3390/nu10121981. Nutrients. 2018. PMID: 30558183 Free PMC article. - The ins and outs of mammary gland calcium and zinc transport: A brief review.
Kelleher SL. Kelleher SL. JDS Commun. 2022 Nov 18;4(3):240-244. doi: 10.3168/jdsc.2022-0291. eCollection 2023 May. JDS Commun. 2022. PMID: 37360130 Free PMC article. Review.
References
- Ackland ML, Mercer JF. The murine mutation, lethal milk, results in production of zinc-deficient milk. J Nutr 122: 1214–1218, 1992. - PubMed
- Brown K. Impact of obesity on mammary gland inflammation and local estrogen production. J Mammary Gland Biol Neoplasia 19: 183–189, 2014. - PubMed
- Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem 281: 39,699–39,707, 2006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous