Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy - PubMed (original) (raw)
. 2015 Nov 27;350(6264):1084-9.
doi: 10.1126/science.aac4255. Epub 2015 Nov 5.
Leticia Corrales 1, Nathaniel Hubert 2, Jason B Williams 1, Keston Aquino-Michaels 3, Zachary M Earley 2, Franco W Benyamin 1, Yuk Man Lei 2, Bana Jabri 2, Maria-Luisa Alegre 2, Eugene B Chang 2, Thomas F Gajewski 4
Affiliations
- PMID: 26541606
- PMCID: PMC4873287
- DOI: 10.1126/science.aac4255
Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy
Ayelet Sivan et al. Science. 2015.
Abstract
T cell infiltration of solid tumors is associated with favorable patient outcomes, yet the mechanisms underlying variable immune responses between individuals are not well understood. One possible modulator could be the intestinal microbiota. We compared melanoma growth in mice harboring distinct commensal microbiota and observed differences in spontaneous antitumor immunity, which were eliminated upon cohousing or after fecal transfer. Sequencing of the 16S ribosomal RNA identified Bifidobacterium as associated with the antitumor effects. Oral administration of Bifidobacterium alone improved tumor control to the same degree as programmed cell death protein 1 ligand 1 (PD-L1)-specific antibody therapy (checkpoint blockade), and combination treatment nearly abolished tumor outgrowth. Augmented dendritic cell function leading to enhanced CD8(+) T cell priming and accumulation in the tumor microenvironment mediated the effect. Our data suggest that manipulating the microbiota may modulate cancer immunotherapy.
Copyright © 2015, American Association for the Advancement of Science.
Figures
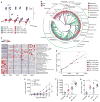
Fig. 1. Differences in melanoma outgrowth and tumor-specific immune responses between C57BL/6 JAX and TAC mice are eliminated when mice are cohoused
(A) B16. SIY tumor growth kinetics in newly arrived JAX and TAC mice. (B) IFN-γ enzyme-linked immunospot assay (ELISPOT) in tumor-bearing JAX and TAC mice 7 days after tumor inoculation. (C) Mean size of IFN-γ spots (10−3 mm2). (D) Percentage of SIY+ T cells of total CD8+ T cells within the tumor of JAX and TAC mice as determined by flow cytometry 21 days after tumor inoculation. Representative plots (left), quantification (right). (E) B16.SIY tumor growth kinetics in JAX and TAC mice cohoused for 3 weeks before tumor inoculation. (F) Number of IFN-γ spots/106 splenocytes in tumor-bearing JAX and TAC mice cohoused for 3 weeks before tumor inoculation. (G) Mean size of IFN-γ spots (10−3 mm2). (H) Percentage of SIY+ T cells of total CD8+ T cells within the tumor of JAX and TAC mice cohoused for 3 weeks before tumor inoculation. Means ± SEM combined from six independent experiments, analyzed by two-way analysis of variance (ANOVA) with Sidak’s correction for multiple comparisons (A) and (E), or individual mice with means ± SEM combined from four (B), (C), (F), (G) or three (D) and (H) independent experiments, analyzed by Student’s t test; five mice per group per experiment; *P < 0.005, **P < 0.01; NS, not significant.
Fig. 2. Oral administration of JAX fecal material to TAC mice enhances spontaneous antitumor immunity and response to αPD-L1 mAb therapy
(A) B16.SIY tumor growth in newly arrived TAC mice, TAC and JAX mice orally gavaged with phosphate-buffered saline or TAC or JAX fecal material before tumor implantation. (B) Number of IFN-γ spots × mean spot size (10−3 mm2), determined by ELISPOT 7 days after tumor inoculation. (C) Percentage of SIY+ CD8+ Tcells within the tumor of TAC and JAX mice treated as in (A), 21 days after tumor inoculation. Representative plots (left), quantification (right). (D) B16.SIY tumor growth in TAC mice, untreated or treated with JAX fecal material 7 and 14 days after tumor implantation, αPD-L1 mAb 7, 10, 13, and 16 days after tumor implantation, or both regimens. (E) IFN-γ ELISPOT assessed 5 days after start of treatment. (F) Percentage of tumor-infiltrating SIY+ CD8+ Tcells, determined by flow cytometry 14 days after start of treatment. (G) B16.SIY tumor growth kinetics in TAC and JAX mice, untreated or treated with αPD-L1 mAb 7, 10, 13, and 16 days after tumor implantation. Means ± SEM analyzed by two-way analysis of variance (ANOVA) with Dunnett’s (A) or Tukey’s (D) and (G) correction for multiple comparisons; or individual mice with means ± SEM analyzed by one-way ANOVA with Holm-Sidak correction for multiple comparisons (B), (C), (E), and (F); data are representative of (A) to (C), (F), and (G) or combined from (D) and (E) two to four independent experiments; five mice per group per experiment; *P < 0.05, **P < 0.01, ****P < 0.0001; NS, not significant.
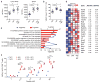
Fig. 3. Direct administration of Bifidobacterium to TAC recipients with established tumors improves tumor-specific immunity and response to αPD-L1 mAb therapy
(A) Principal coordinate analysis plot of bacterial β-diversity over time in groups treated as in Fig. 2A, each group is made up of at least two cages, three or four mice per cage; data represent three independent experiments; **P < 0.01, ***P < 0.001 (ANOSIM). (B) Phylogenetic analysis of taxa that are of significantly different abundance in newly arrived JAX versus TAC mice FDR < 0.05 (non-parametric t test); bars represent log-transformed fold changes, inner circle, log10(10); middle circle, log10(100); outer circle, log10(1000). (C) Heat map showing relative abundance over time of significantly altered genus-level taxa in JAX-fed TAC mice FDR < 0.05 (nonparametric t test); columns depict individual mice; each time point shows mice from two separate cages, three or four mice per cage. (D) Correlation plot of relative abundance of Bifidobacterium OTU_681370 in fecal material obtained from groups, as in (A), 14 days after arrival and frequency of SIY+ CD8+ T cells in tumor; P = 1.4 × 10−5, FDR = 0.0002, correlation _R_2 = 0.86 (univariate regression). (E) B16.SIY tumor growth kinetics in TAC mice, untreated or treated with Bifidobacterium 7 and 14 days after tumor implantation, αPD-L1 mAb 7, 10, 13, and 16 days after tumor implantation, or both regimens. (F) IFN-γ ELISPOT assessed 5 days after start of treatment. (G) Percentage of tumor-infiltrating SIY+ CD8+ Tcells, determined by flow cytometry 14 days after start of treatment. Means ± SEM analyzed by two-way ANOVA with Tukey’s correction (E) or individual mice with means ± SEM analyzed by one-way ANOVA with Holm-Sidak correction (F) and (G), and are combined from two independent experiments; five mice per group per experiment: *P < 0.05, ***P < 0.001, ****P < 0.0001.
Fig. 4. Dendritic cells isolated from JAX and _Bifidobacterium_-fed TAC mice show increased expression of genes associated with antitumor immunity and heightened capability for Tcell activation
(A) Quantification of IFN-γ mean fluorescence intensity (MFI) of 2C CD8+ Tcells in the tumor-draining lymph node (left) and spleen (right) of TAC, JAX, and _Bifidobacterium_-fed TAC mice on day 7 after adoptive transfer. (B) Percentage of MHC Class IIhi DCs in tumors isolated from TAC, JAX, and _Bifidobacterium_-fed TAC mice 40 hours after tumor implantation as assessed by flow cytometry. Data in (A) and (B) show individual mice with means ± SEM, analyzed by one-way ANOVA with Holm-Sidak correction; representative of two to four independent experiments, eight or nine mice per group per experiment: *P < 0.05, **P < 0.01, ****P < 0.0001. (C) Enriched biological pathways and functions found within the subset of elevated genes in JAX and _Bifidobacterium_-treated TAC-derived DCs relative to untreated TAC DCs isolated from tumors 40 hours after tumor inoculation, as assessed by DAVID pathway analysis. Red bars indicate the percentage of genes in a pathway up-regulated in DCs isolated from JAX and _Bifidobacterium_-fed TAC mice. Blue line indicates P values calculated by Fisher’s exact test. (D) Heat map of key antitumor immunity genes in DCs isolated from JAX, _Bifidobacterium_-treated TAC or untreated TAC mice. Mean fold-change for each gene transcript is shown on the right. (E) Quantification of IFN-γ+ 2C TCR Tg CD8+ Tcells stimulated in vitro with DCs purified from peripheral lymphoid tissues of naïve TAC, JAX, and _Bifidobacterium_-treated TAC mice in the presence of different concentrations of SIY peptide. Analyses in (C) to (E) were performed on data combined from two independent experiments, five mice pooled per group per experiment. (E) Technical replicates of pooled samples from each experiment separately and were analyzed by fitting a linear mixed model, with Bonferroni correction for multiple comparisons: *P < 0.05, ****P < 0.0001.
Comment in
- IMMUNOTHERAPY. Could microbial therapy boost cancer immunotherapy?
Snyder A, Pamer E, Wolchok J. Snyder A, et al. Science. 2015 Nov 27;350(6264):1031-2. doi: 10.1126/science.aad7706. Science. 2015. PMID: 26612936 No abstract available. - Tumour immunology: Intestinal bacteria are in command.
Alderton GK. Alderton GK. Nat Rev Immunol. 2016 Jan;16(1):5. doi: 10.1038/nri.2015.13. Epub 2015 Dec 14. Nat Rev Immunol. 2016. PMID: 26655627 No abstract available. - Immunotherapy Not Working? Check Your Microbiota.
West NR, Powrie F. West NR, et al. Cancer Cell. 2015 Dec 14;28(6):687-689. doi: 10.1016/j.ccell.2015.11.010. Cancer Cell. 2015. PMID: 26678336
Similar articles
- The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients.
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. Matson V, et al. Science. 2018 Jan 5;359(6371):104-108. doi: 10.1126/science.aao3290. Science. 2018. PMID: 29302014 Free PMC article. - Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota.
Dong M, Meng Z, Kuerban K, Qi F, Liu J, Wei Y, Wang Q, Jiang S, Feng M, Ye L. Dong M, et al. Cell Death Dis. 2018 Oct 10;9(10):1039. doi: 10.1038/s41419-018-1099-3. Cell Death Dis. 2018. PMID: 30305604 Free PMC article. - Immunotherapy Not Working? Check Your Microbiota.
West NR, Powrie F. West NR, et al. Cancer Cell. 2015 Dec 14;28(6):687-689. doi: 10.1016/j.ccell.2015.11.010. Cancer Cell. 2015. PMID: 26678336 - Biomarqueurs prédictifs de réponse aux traitements bloquant les voies de costimulation inhibitrices.
Pagès F, Granier C, Kirilovsky A, Elsissy C, Tartour E. Pagès F, et al. Bull Cancer. 2016 Nov;103 Suppl 1:S151-S159. doi: 10.1016/S0007-4551(16)30373-3. Bull Cancer. 2016. PMID: 28057179 Review. French. - Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies.
Philips GK, Atkins M. Philips GK, et al. Int Immunol. 2015 Jan;27(1):39-46. doi: 10.1093/intimm/dxu095. Epub 2014 Oct 16. Int Immunol. 2015. PMID: 25323844 Review.
Cited by
- Microbiota activation and regulation of adaptive immunity.
Heidari M, Maleki Vareki S, Yaghobi R, Karimi MH. Heidari M, et al. Front Immunol. 2024 Oct 9;15:1429436. doi: 10.3389/fimmu.2024.1429436. eCollection 2024. Front Immunol. 2024. PMID: 39445008 Free PMC article. Review. - Acute Radiation Syndrome and the Microbiome: Impact and Review.
Hollingsworth BA, Cassatt DR, DiCarlo AL, Rios CI, Satyamitra MM, Winters TA, Taliaferro LP. Hollingsworth BA, et al. Front Pharmacol. 2021 May 18;12:643283. doi: 10.3389/fphar.2021.643283. eCollection 2021. Front Pharmacol. 2021. PMID: 34084131 Free PMC article. Review. - Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment.
Wu H, Ganguly S, Tollefsbol TO. Wu H, et al. Microorganisms. 2022 Aug 27;10(9):1727. doi: 10.3390/microorganisms10091727. Microorganisms. 2022. PMID: 36144329 Free PMC article. Review. - Probiotic and Antioxidant Potential of _Lactobacillus reuteri_LR12 and _Lactobacillus lactis_LL10 Isolated from Pineapple Puree and Quality Analysis of Pineapple-Flavored Goat Milk Yoghurt during Storage.
Al-Dhabi NA, Valan Arasu M, Vijayaraghavan P, Esmail GA, Duraipandiyan V, Kim YO, Kim H, Kim HJ. Al-Dhabi NA, et al. Microorganisms. 2020 Sep 23;8(10):1461. doi: 10.3390/microorganisms8101461. Microorganisms. 2020. PMID: 32977600 Free PMC article. - Probiotic-derived p8 protein induce apoptosis via regulation of RNF152 in colorectal cancer cells.
Kim BK, Yoon YS, Ryu Y, Chung MJ. Kim BK, et al. Am J Cancer Res. 2021 Mar 1;11(3):746-759. eCollection 2021. Am J Cancer Res. 2021. PMID: 33791151 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK042086/DK/NIDDK NIH HHS/United States
- T32 CA009594/CA/NCI NIH HHS/United States
- T32 AI007090/AI/NIAID NIH HHS/United States
- R01 AI115716/AI/NIAID NIH HHS/United States
- P30 DK42086/DK/NIDDK NIH HHS/United States
- 5T32CA009594-25/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials