Multitargeting activity of miR-24 inhibits long-term melatonin anticancer effects - PubMed (original) (raw)
. 2016 Apr 12;7(15):20532-48.
doi: 10.18632/oncotarget.7978.
Maria Ferraiuolo 1 2, Raffaela Santoro 1, Andrea Sacconi 2, Frauke Goeman 2, Matteo Pallocca 3, Claudio Pulito 1, Etleva Korita 1, Maurizio Fanciulli 3, Paola Muti 4, Giovanni Blandino 2 4, Sabrina Strano 1 4
Affiliations
- PMID: 26967561
- PMCID: PMC4991473
- DOI: 10.18632/oncotarget.7978
Multitargeting activity of miR-24 inhibits long-term melatonin anticancer effects
Federica Mori et al. Oncotarget. 2016.
Abstract
We have previously shown that melatonin exerts tumor suppressor activities by inducing the p38-p53 axis. This occurred within a few hours while no data are available on how melatonin pathway can be sustained on the long term. Here we show that miR-24, which has been demonstrated to target genes involved in the DNA repair process, targets p38, p53, PML and H2AX simultaneously. We show that long-term treatment with melatonin can decrease miR-24 levels post-transcriptionally, which pairs with a long-wave regulation of genes involved in cell proliferation, DNA damage, RNA metabolism and cell shape and transformation. Moreover, we show that melatonin can inhibit cell proliferation and migration, at least in part, by downregulating miR-24. Furthermore, we propose the involvement of hnRNP A1, which is downregulated by melatonin and involved in miRNA processing, in the regulation of miR-24 levels by melatonin. We conclude showing that miR-24 is upregulated in colon, breast and head and neck datasets and its levels negatively correlate with overall survival.
Keywords: PML; RNA-Seq; melatonin; miR-24; p53.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Figures
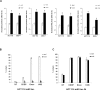
Figure 1. miR-24 targets the melatonin-p53 pathway
(A) Graphs show the sequence coverage, as well as the signal intensity, of H2AFX (H2AX) and MAPK12 (p38-γ) in both vehicle and melatonin treated HCT 116 cell line. (B) HCT 116 and MCF-7 cells have been treated with melatonin for 24, 48 and 72 hours. The ratio between miR-24 and RNU49 levels, normalized to their respective untreated controls, are indicated in the graphs. (C) HCT 116 cells have been transfected with either negative control (NC) or miR-24 mimic and the indicated Dual Luciferase reporters. Values for NC are set to 1. *p < 0,01. (D, E) HCT 116 and MCF7 cells have been transfected with the indicated mimic (D) or LNA (E) and subjected to quantitative Real-Time PCR. Values have been normalized to NC or LNA. (F, G) Cells have been transfected with the indicated mimic (F) or LNA (G) and cell extracts have been subjected to immunoblot with the indicated antibodies. Numbers indicate signal quantification normalized to NC or LNA-NC.
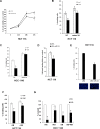
Figure 2. Melatonin induces the expression of the p53-pathway and alleviates genomic instability caused by miR-24
(A) HCT 116 and MCF-7 cell lines have been treated or not with melatonin for 72 hours and the expression levels of the indicated mRNAs were assessed by quantitative Real-Time PCR. Values have been normalized to untreated control. *p < 0,01. (B) HCT 116 cell lines stably expressing miR-Vec or (C) miR-Vec-24 have been pre-treated with either vehicle or melatonin, then DNA damage has been induced as indicated (CDDP: Cisplatin, Doxo: Doxorubicin, UVB: Ultra Violet B rays). Cells have been subjected to comet assay. Histograms show the percentage of comets.
Figure 3. Melatonin impairs the capability of miR-24 to induce cell proliferation and migration
(A) Cells were transfected with the indicated LNA and allowed to proliferate. Percentage of cells relative to control is indicated in the graph. *p < 0,05. (B) HCT 116 and (C) HCC1143 cells were transiently transfected with either NC or mimic-24 and subjected to transwell migration assays in the absence and in the presence of melatonin. Histograms show the percentage of invading cells. *p < 0,001. (D) HCT 116 cells were transfected with the indicated LNA and subjected to wound healing assay. Histograms show the percentage of wound opening. *p < 0,05. (E) HCT 116 were transfected with the indicated LNA and subjected to transwell migration assay. Histograms show the percentage of invading cells. *p < 0,01. (F) HCT 116 cells have been treated with 5 μM SB202190 for 2 hours and then subjected to transwell migration assay. *p < 0,05. (G) HCC1143 cells have been subjected to wound healing assay in the absence and in the presence of SB202190 and melatonin. Histograms show the percentage of wound opening. *p < 0,05.
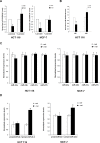
Figure 4. Regulation of miR-24 levels by melatonin
(A) HCT 116 and MCF-7 cells have been either pretreated or not with Luzindole 15 minutes before melatonin treatment and miR-24 levels have been quantified 72 h later by quantitative Real-Time PCR. Histograms show miR-24/RNU49 levels relative to untreated control. *p < 0,01. (B) HCT 116 cells have been treated with melatonin for 72 hours in the absence and in the presence of 5 μM SB202190. Histograms show miR-24/RNU49 levels relative to untreated control. *p < 0,01. (C) HCT 116 and MCF-7 cells were treated with melatonin for 72 h. Levels of expression of the indicated miRNAs were assessed by quantitative Real-Time PCR. (D) HCT 116 and MCF-7 cells were treated with melatonin for 72 h. Histograms show the ratio between pre-miR-24–1 and pri-miR-24–1 and the ratio between pre-miR-24–2 and pri-miR-24–2 levels. *p < 0,01.
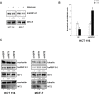
Figure 5. hnRNP A1 is involved in the regulation of miR-24 levels
(A) HCT 116 and MCF-7 cells were treated with melatonin for 72 h and protein extracts were subjected to immunoblot with the indicated antibodies. Numbers indicate the densitometry ratio between hnRNP A1 and 20Sα5 signals. (B) HCT 116 cells were transfected with the indicated plasmids and treated with melatonin for 72 h, miR-24 levels were assessed by quantitative Real-Time PCR. *p < 0,01. (C) HCT 116 and MCF-7 were transfected with the indicated siRNAs. 72 h post transfection, cell lysates were subjected to immunoblot with the indicated antibodies. Numbers indicate the densitometry ratio between hnRNP A1 and tubulin or nucleolin signals, normalized to siGFP.
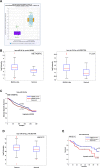
Figure 6. Clinical association of miR-24 with survival and recurrence in cancer patients
(A) Box plot showing miR-24 median value in 314 cancer cases and 11 controls belonging to the Pan-Cancer Colon and rectal adenocarcinoma datasets. (B-C) Box plot showing miR-24 median value in 208 basal-like cancers and 116 controls (METABRIC) and 90 basal-like cancers and 83 controls (TCGA) (B) and association between expression levels of miR-24 and disease-free survival evaluated by Kaplan-Meier analysis in the METABRIC dataset, comprising 1284 cases and 116 controls (C). (D–E) Box plot showing miR-24 median value in 121 cancer cases and 66 controls (D) and association between expression levels of miR-24 and recurrence-free survival evaluated by Kaplan-Meier analysis (E) in our HNSCC casuistry [61].
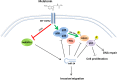
Figure 7. Model of melatonin activities
By binding to its receptors (MT1/MT2), Melatonin induces p38 expression. With a fast kinetics (green arrow), Melatonin induces phosphorylation of p53 and H2AX thereby promoting DNA repair and inhibiting cell proliferation. Long-term activation of Melatonin receptors sustains p38 activation and leads to inhibition of hnRNP A1 (red arrow) thereby causing a decrease in miR-24, which results in p53, PML, H2AX and p38 activity reduction. Through downregulation of hnRNP A1 and miR-24, melatonin impairs the migrating capability of cancer cells.
Similar articles
- MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1.
Zheng M, Sun X, Li Y, Zuo W. Zheng M, et al. Tumour Biol. 2016 Jun;37(6):8189-96. doi: 10.1007/s13277-015-4722-2. Epub 2015 Dec 29. Tumour Biol. 2016. PMID: 26715279 - Tumor-suppressive microRNA-34a inhibits breast cancer cell migration and invasion via targeting oncogenic TPD52.
Li G, Yao L, Zhang J, Li X, Dang S, Zeng K, Zhou Y, Gao F. Li G, et al. Tumour Biol. 2016 Jun;37(6):7481-91. doi: 10.1007/s13277-015-4623-4. Epub 2015 Dec 17. Tumour Biol. 2016. PMID: 26678891 - Long non-coding RNA ZFAS1 interacts with miR-150-5p to regulate Sp1 expression and ovarian cancer cell malignancy.
Xia B, Hou Y, Chen H, Yang S, Liu T, Lin M, Lou G. Xia B, et al. Oncotarget. 2017 Mar 21;8(12):19534-19546. doi: 10.18632/oncotarget.14663. Oncotarget. 2017. PMID: 28099946 Free PMC article. - miR-203 reverses chemoresistance in p53-mutated colon cancer cells through downregulation of Akt2 expression.
Li J, Chen Y, Zhao J, Kong F, Zhang Y. Li J, et al. Cancer Lett. 2011 May 1;304(1):52-9. doi: 10.1016/j.canlet.2011.02.003. Epub 2011 Feb 26. Cancer Lett. 2011. PMID: 21354697 - MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer.
Vilquin P, Donini CF, Villedieu M, Grisard E, Corbo L, Bachelot T, Vendrell JA, Cohen PA. Vilquin P, et al. Breast Cancer Res. 2015 Jan 30;17(1):13. doi: 10.1186/s13058-015-0515-1. Breast Cancer Res. 2015. PMID: 25633049 Free PMC article.
Cited by
- Genome-Protecting Compounds as Potential Geroprotectors.
Proshkina E, Shaposhnikov M, Moskalev A. Proshkina E, et al. Int J Mol Sci. 2020 Jun 24;21(12):4484. doi: 10.3390/ijms21124484. Int J Mol Sci. 2020. PMID: 32599754 Free PMC article. Review. - MiR-24-3p as a prognostic indicator for multiple cancers: from a meta-analysis view.
Wang H, Chen C, Ding K, Zhang W, Hou J. Wang H, et al. Biosci Rep. 2020 Dec 23;40(12):BSR20202938. doi: 10.1042/BSR20202938. Biosci Rep. 2020. PMID: 33206184 Free PMC article. - The Impact of miRNA in Colorectal Cancer Progression and Its Liver Metastases.
Balacescu O, Sur D, Cainap C, Visan S, Cruceriu D, Manzat-Saplacan R, Muresan MS, Balacescu L, Lisencu C, Irimie A. Balacescu O, et al. Int J Mol Sci. 2018 Nov 22;19(12):3711. doi: 10.3390/ijms19123711. Int J Mol Sci. 2018. PMID: 30469518 Free PMC article. Review. - Overexpression of microRNA‑101 causes anti‑tumor effects by targeting CREB1 in colon cancer.
Yang Q, Yu W, Han X. Yang Q, et al. Mol Med Rep. 2019 Apr;19(4):3159-3167. doi: 10.3892/mmr.2019.9952. Epub 2019 Feb 14. Mol Med Rep. 2019. PMID: 30816471 Free PMC article. - RNA-Seq transcriptome analysis shows anti-tumor actions of melatonin in a breast cancer xenograft model.
Jardim-Perassi BV, Alexandre PA, Sonehara NM, de Paula-Junior R, Reis Júnior O, Fukumasu H, Chammas R, Coutinho LL, Zuccari DAPC. Jardim-Perassi BV, et al. Sci Rep. 2019 Jan 30;9(1):966. doi: 10.1038/s41598-018-37413-w. Sci Rep. 2019. PMID: 30700756 Free PMC article.
References
- Aoki H, Ozeki Y, Yamada N. Hypersensitivity of melatonin suppression in response to light in patients with delayed sleep phase syndrome. Chronobiology international. 2001;18:263–271. - PubMed
- Carbajo-Pescador S, Garcia-Palomo A, Martin-Renedo J, Piva M, Gonzalez-Gallego J, Mauriz JL. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. Journal of pineal research. 2011;51:463–471. - PubMed
- Cuesta S, Kireev R, Forman K, Garcia C, Escames G, Ariznavarreta C, Vara E, Tresguerres JA. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8) Experimental gerontology. 2010;45:950–956. - PubMed
- Jones CR, Campbell SS, Zone SE, Cooper F, DeSano A, Murphy PJ, Jones B, Czajkowski L, Ptacek LJ. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nature medicine. 1999;5:1062–1065. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous