The secretome of periodontal ligament stem cells from MS patients protects against EAE - PubMed (original) (raw)
The secretome of periodontal ligament stem cells from MS patients protects against EAE
Thangavelu Soundara Rajan et al. Sci Rep. 2016.
Abstract
Manipulation of stem cells or stem cells-derived secretome has emerged as a novel alternative therapeutic option for multiple sclerosis (MS). Here we show that human periodontal ligament stem cells (hPDLSCs)-derived conditioned medium (hPDLSCs-CM) and purified exosomes/microvesicles (hPDLSCs-EMVs) obtained from Relapsing Remitting (RR)-MS patients and healthy donors block experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, by inducing anti-inflammatory and immunosuppressive effects in spinal cord and spleen, and reverse disease progression by restoring tissue integrity via remyelination in the spinal cord. We show that hPDLSCs-CM and hPDLSCs-EMVs reduce pro-inflammatory cytokines IL-17, IFN-γ, IL-1β, IL-6, TNF-α, and induce anti-inflammatory IL-10. In addition, apoptosis related STAT1, p53, Caspase 3, and Bax expressions were attenuated. Our findings unravel the immunosuppressive effects of hPDLSCs-CM and hPDLSCs-EMVs in EAE mice, and suggest simple alternative autologous source for patient-customized cell-free targeting treatment in MS patients.
Figures
Figure 1. Flow cytometry of hPDLSCs and RR-MS-hPDLSCs phenotypes.
Flow cytometry phenotype of surface related antigen of hPDLSCs and RR-MS-hPDLSCs at the 2nd passage (CD13, CD14, CD29, CD31, CD34, CD44, CD45, CD73, CD90, CD105, CD106, CD117, CD133, CD144, CD146, CD166, CD326, HLA-ABC, HLA-DR) and intracellular stemness (SSEA4, Oct3/4, Sox2, NANOG) marker expression levels were detected. Red histograms show the distribution of each antigen expression, whereas Blue histograms represent the distribution of the respective background control. Data are representative of five separate experiments.
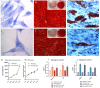
Figure 2. Toluidine blue staining of primary cultures of hPDLSCs observed by light microscopy.
The cells display a fibroblast-like appearance with long cytoplasmatic processes, euchromatic nuclei with one or more nucleoli and rough endoplasmic reticulum profiles in both hPDLSCs [A] and RR-MS-hPDLSCs [D] samples. Original magnification: 40X. Cell proliferation and viability are assessed by Trypan blue exclusion test and MTT assay respectively [G and H]. The results are expressed as mean ± SEM of five independent experiments, and five replicates for each experimental point. hPDLSCs, and RR-MS-hPDLSCs induced to osteogenic differentiation were stained with Alizarin Red S after 3 weeks of culture [B and E, respectively]. Insets display uninduced hPDLSCs from healthy donors [b1] and RR-MS patients [e1]; b2 and e2 show differentiated hPDLSCs and RR-MS-hPDLSCs, respectively. High levels of mineralization are evident in the above mentioned samples. Original magnification: 10×. The bar graph shows mRNA levels, determined by real-time PCR, of osteo-related genes, i.e., alkaline phosphatase (ALP) and Runt-related transcription factor-2 (RUNX2) at 7 days of culture [I]. Adipogenic differentiation has been evaluated by the appearance of oil-red O-positive lipid vacuoles. [C and F, magnification: 40X]. The adipo-related genes, i.e., fatty acid binding protein 4 (FABP4) and peroxisome proliferator-activated receptor γ (PPARγ), analyzed by real-time PCR are shown [J]. The related mesengenic differentiation gene were over expressed in similar manner in hPDLSCs, and RR-MS- hPDLSCs. **p < 0.05. Scale bars = 10 μm. For each experiment, a representative image has been shown.
Figure 3. Characterization of EMVs detected in hPDLSCs and RR-MS-hPDLSCs conditioned medium.
Filled histograms show the distribution of CD29 and CD90 antigen expression, whereas open histograms represent the distribution of the respective background control in hPDLSCs [A and B] and RR-MS-hPDLSCs [C and D]. The expression of CD63 was evaluated in combination with Mitotracker to exclude debris [E and F]. Data are representative of five separate experiments. The characterization of isolated EMVs stained with WGA derived from hPDLSCs and RR-MS-hPDLSCs is reported in section [G and H, respectively]. Different size in the parental EMVs isolated with the ExoQuick-TC method was observed. Scale bars: 5 μM.
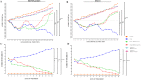
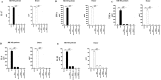
Figure 4. Body weight and clinical score.
Mice were immunized with MOG 35–55 and monitored 28 days for body weight gain/loss and clinical disease score. (A and B) The variation of body weight has been expressed compared to day of EAE induction (day 0) for each experimental group. Naive mice showed a normal increase in body weight. On the contrary, a significant body weight loss was observed in EAE mice, whereas a relevant body weight gain was found in EAE mice treated with RR-MS patients human periodontal ligament stem cells (hPDLSCs)-derived conditioned medium (hPDLSCs-CM) or purified exosomes/microvesicles (hPDLSCs-EMVs) [A; ****p < 0.0001 vs EAE; **p = 0.0029 vs EAE + hPDLSCs-CM; **p = 0.0030 vs EAE + hPDLSCs-EMVs] and in EAE mice treated with donors hPDLSCs-CM or hPDLSCs-EMVs [B; ****p < 0.0001 vs EAE; **p = 0.0025 vs EAE + hPDLSCs-CM; **p = 0.0026 vs EAE + hPDLSCs-EMVs]. (C and D) Naive mice did not display motor deficit. EAE mice displayed a grading of disease, while significant reduction in the clinical scores was observed in EAE mice treated with RR-MS patients hPDLSCs-CM or hPDLSCs-EMVs [C; ****p < 0.0001 vs EAE; ***p < 0.0009 vs EAE; ****p < 0.0001 vs naive] and in EAE mice treated with donors hPDLSCs-CM or hPDLSCs-EMVs [D; ***p < 0.0008 vs EAE; ***p < 0.0002 vs EAE; ****p < 0.0001 vs naive].
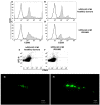
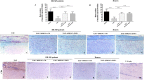
Figure 5. Golgi staining, LFB staining and CD4 expression in the spinal cord.
Dendritic spines were reduced in EAE mice, whereas EAE mice treated with hPDLSCs-CM or hPDLSCs-EMVs obtained from RR-MS patients [A] and donors [B] showed significant augmentation in spine density. In addition, EAE mice without treatment exhibited remarkably reduced myelin in the spinal cord [C; area showed in dotted square], whereas treatment with hPDLSCs-CM or hPDLSCs-EMVs obtained from RR-MS patients [D and E, respectively] and donors [F and G, respectively] reduced demyelination and axonal loss in EAE mice with an intense LFB positive staining. CD4 [H] expression revealed marked positive staining in EAE mice, while EAE mice administered with hPDLSCs-CM or hPDLSCs-EMVs derived from MS patients [I and J, respectively] and donors [K and L, respectively] showed negative staining. [M] only secondary antibody.
Figure 6. ELISA assay for inflammatory cytokines in the spinal cord.
ELISA data demonstrated that proinflammatory cytokines IL-17 [A], IFN-γ [B], TNF-α [C], IL-6 [D] and IL-1β [E] were markedly increased in EAE mice, while administration of RR-MS patients and donors derived hPDLSCs-CM and hPDLSCs-EMVs significantly reduced their expression. The data are representative of three independent experiments. Error bars indicate mean ± SD. ####p < 0.0001.
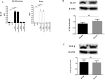
Figure 7. ELISA assay for IL-10 in the spinal cord and Western blot analysis for IL-10 and TGF-β in hPDLSCs-EMVs.
Anti-inflammatory IL-10 was undetectable in EAE mice, while administration of hPDLSCs-CM or hPDLSCs-EMVs derived from RR-MS patients and donors siginificantly augmented IL-10 expression [A]. The data are representative of three independent experiments. Error bars indicate mean ± SD. ####p < 0.0001. Western blot analysis in the extract of hPDLSCs-EMVs isolated from healthy donors and RR-MS patients showed a marked level of IL-10 [B] and TGF-β [C]. IL-10 and TGF-β were quantified by densitometry after normalizing the bands to β-actin. No statistical significance in IL-10 and TGF-β expression was noticed between RR-MS patients and donors hPDLSCs-EMVs. A representative graph of three independent experiments is shown. ns-no statistical significance.
Figure 8. Western blot analysis for STAT1, p53, and cleaved caspase 3, and immunohistochemical evaluation for Bax in the spinal cord.
Western blot analysis showed a significant expression of proapoptotic STAT1 [A and D] and p53 [B and E] in EAE mice. Conversely, levels of STAT1 and p53 were significantly reduced by administration of hPDLSCs-CM or hPDLSCs-EMVs derived from MS patients and donors. A **p < 0.0011 vs naive, **p < 0.0070 vs CM, **p < 0.0093 vs EMVs. D ****p < 0.0001. B **p < 0.0047 vs naive, **p < 0.0056 vs CM, **p < 0.0081 vs EMVs. E ****p < 0.0001. Moreover, EAE caused a significant increase in proapoptotic cleaved caspase 3 expression. On the contrary, treatment with hPDLSCs-CM or hPDLSCs-EMVs derived from MS patients [C] and donors [F] significantly prevented the EAE-induced cleaved caspase 3 expression. C ****p < 0.0001. F ****p < 0.0001. STAT1, p53 and cleaved caspase 3 were quantified by densitometry after normalizing the bands to GAPDH or caspase 3. Representative bands of three independent experiments are shown. Error bars indicate mean ± SD. ND: not detectable. Immunohistochemical evaluation revealed an increased Bax tissue localization in EAE mice [G]. On the contrary, negative staining of Bax was observed in mice that received hPDLSCs-CM or hPDLSCs-EMVs derived from MS patients [H and I, respectively] and donors [J and K, respectively]. Magnification 10X. L only secondary antibody.
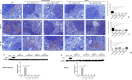
Figure 9. Modulation of inflammatory cytokines in the spleen.
Immunohistochemistry analyses of pro-inflammatory IL-17 [A; densitometric analysis F] and IFN-γ [G; densitometric analysis L] revealed higher tissue levels of these markers in EAE mice, while significant reduction was noticed in EAE mice administered with hPDLSCs-CM or hPDLSCs-EMVs derived from RR-MS patients [B,C and H,I, respectively] and donors [D,E and J,K, respectively]. EAE sections did not stain for anti-inflammatory IL-10 antibody [M; densitometric analysis R], while significant positive IL-10 staining was noticed in the EAE group treated with hPDLSCs-CM or hPDLSCs-EMVs obtained from MS patients [N and O, respectively] and donors [P and Q, respectively]. ****p < 0.0001 vs EAE. Magnification 40X. Western blot analysis showed a significant expression of pro-inflammatory CD4 in the spleen of EAE mice [S and T, respectively]. Conversely, as in naive animals, CD4 was undetectable in the EAE group treated with hPDLSCs-CM or hPDLSCs-EMVs obtained from RR-MS patients and donors. CD4 was quantified by densitometry after normalizing the bands to GAPDH. Representative bands of three independent experiments are shown. Error bars indicate mean ± SD. ****p < 0.0001.
Similar articles
- Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis.
Soundara Rajan T, Giacoppo S, Diomede F, Bramanti P, Trubiani O, Mazzon E. Soundara Rajan T, et al. Int J Immunopathol Pharmacol. 2017 Sep;30(3):238-252. doi: 10.1177/0394632017722332. Epub 2017 Aug 2. Int J Immunopathol Pharmacol. 2017. PMID: 28764573 Free PMC article. - Anti-inflammatory effects of hypoxia-preconditioned human periodontal ligament cell secretome in an experimental model of multiple sclerosis: a key role of IL-37.
Giacoppo S, Thangavelu SR, Diomede F, Bramanti P, Conti P, Trubiani O, Mazzon E. Giacoppo S, et al. FASEB J. 2017 Dec;31(12):5592-5608. doi: 10.1096/fj.201700524R. Epub 2017 Aug 23. FASEB J. 2017. PMID: 28842429 Free PMC article. - Conditioned medium from relapsing-remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS-gingivalis in THP-1 and MO3.13 cell lines.
Ballerini P, Diomede F, Petragnani N, Cicchitti S, Merciaro I, Cavalcanti MFXB, Trubiani O. Ballerini P, et al. Cytokine. 2017 Aug;96:261-272. doi: 10.1016/j.cyto.2017.04.022. Epub 2017 May 13. Cytokine. 2017. PMID: 28511117 - Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE).
Barclay W, Shinohara ML. Barclay W, et al. Brain Pathol. 2017 Mar;27(2):213-219. doi: 10.1111/bpa.12477. Brain Pathol. 2017. PMID: 27997058 Free PMC article. Review. - Effect of Mechanical Force Stress on the Inflammatory Response in Human Periodontal Ligament Cells.
Rojasawasthien T, Srithanyarat SS, Bulanawichit W, Osathanon T. Rojasawasthien T, et al. Int Dent J. 2025 Feb;75(1):117-126. doi: 10.1016/j.identj.2024.12.001. Epub 2024 Dec 26. Int Dent J. 2025. PMID: 39730290 Free PMC article. Review.
Cited by
- Stem Cells Secretome from Oral Tissue Could Represent a Promising Therapeutic Approach in COVID-19-Disease?
Diomede F, Marconi GD, Fonticoli L, Pizzicannella J, Trubiani O. Diomede F, et al. Int J Mol Sci. 2020 Sep 17;21(18):6833. doi: 10.3390/ijms21186833. Int J Mol Sci. 2020. PMID: 32957696 Free PMC article. - The Role of Hypoxia on the Neuronal Differentiation of Gingival Mesenchymal Stem Cells: A Transcriptional Study.
Gugliandolo A, Diomede F, Scionti D, Bramanti P, Trubiani O, Mazzon E. Gugliandolo A, et al. Cell Transplant. 2019 May;28(5):538-552. doi: 10.1177/0963689718814470. Epub 2019 Jan 14. Cell Transplant. 2019. PMID: 30642188 Free PMC article. - Advances in oral mesenchymal stem cell-derived extracellular vesicles in health and disease.
Luo H, Birjandi AA, Ren F, Sun T, Sharpe PT, Sun H, An Z. Luo H, et al. Genes Dis. 2023 Apr 12;11(1):346-357. doi: 10.1016/j.gendis.2023.03.015. eCollection 2024 Jan. Genes Dis. 2023. PMID: 37588220 Free PMC article. Review. - Strategic Tools in Regenerative and Translational Dentistry.
Tatullo M, Codispoti B, Paduano F, Nuzzolese M, Makeeva I. Tatullo M, et al. Int J Mol Sci. 2019 Apr 16;20(8):1879. doi: 10.3390/ijms20081879. Int J Mol Sci. 2019. PMID: 30995738 Free PMC article. Review. - Stemness Maintenance Properties in Human Oral Stem Cells after Long-Term Passage.
Diomede F, Rajan TS, Gatta V, D'Aurora M, Merciaro I, Marchisio M, Muttini A, Caputi S, Bramanti P, Mazzon E, Trubiani O. Diomede F, et al. Stem Cells Int. 2017;2017:5651287. doi: 10.1155/2017/5651287. Epub 2017 Apr 2. Stem Cells Int. 2017. PMID: 28469672 Free PMC article.
References
- Cavaletti G. Current status and future prospective of immunointervention in multiple sclerosis. Curr Med Chem 13, 2329–2343 (2006). - PubMed
- Zappia E. et al.. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106, 1755–1761 (2005). - PubMed
- Kassis I., Petrou P., Halimi M. & Karussis D. Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors. Immunology letters 154, 70–76 (2013). - PubMed
- Constantin G. et al.. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 27, 2624–2635 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous