Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma - PubMed (original) (raw)
Randomized Controlled Trial
Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma
Michele Miraglia Del Giudice et al. Ital J Pediatr. 2017.
Abstract
Background: Allergic rhinitis (AR) and allergic asthma are caused by an IgE-mediated inflammatory reaction. Probiotics may exert anti-inflammatory and immune-modulatory activity. Thus, this study aimed at investigating whether a Bifidobacteria mixture could be able to relieve nasal symptoms, and affect quality of life (QoL) in children with AR and intermittent asthma due to Parietaria allergy.
Materials and methods: The present study was conducted as placebo-controlled, double-blinded, and randomized. Globally, 40 children (18 males; mean age 9 ± 2.2 years) were enrolled. They were treated with probiotics or placebo: 1 sachet/day for 4 weeks. AR symptoms, and QoL were assessed at baseline and after treatment. Use of rescue medications, such as cetirizine syrup and salbutamol spray, was also permitted and recorded.
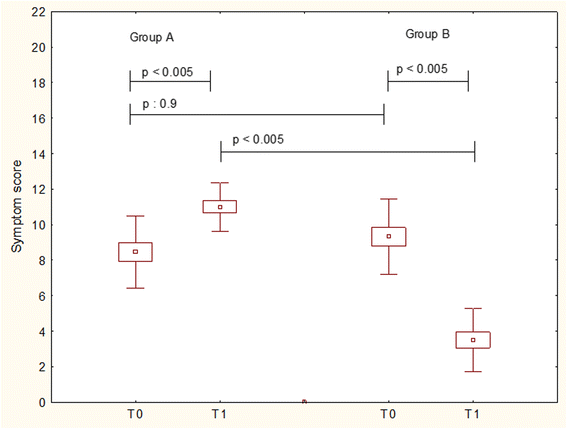
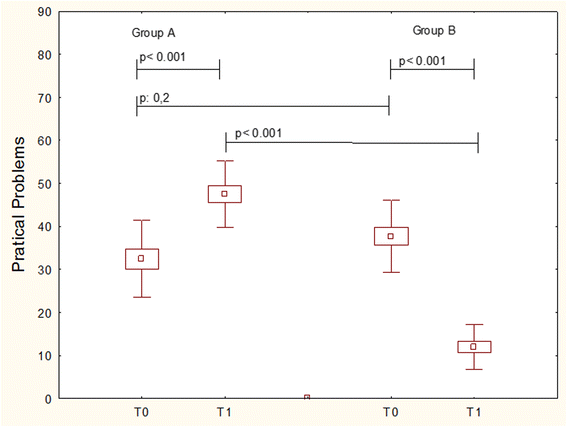
Results: Children treated with probiotic mixture achieved a significant improvement of symptoms (p < 0.005), and QoL ((p < 0.001). Placebo group had worsening of symptoms (p < 0.005) and QoL (p < 0.001). The use of rescue medications was overlapping in the two groups. The intergroup analysis showed that probiotic mixture was significantly superior than placebo for all parameters.
Conclusions: The current study demonstrated that a Bifidobacteria mixture was able of significantly improving AR symptoms and QoL in children with pollen-induced AR and intermittent asthma.
Clinical trial registration: ClinicalTrials.gov ID NCT02807064 .
Keywords: Allergic rhinitis; Bifidobacteria; Children; Intermittent asthma; Probiotic; Quality of life.
Figures
Fig. 1
Total symptom scores in patients treated with Placebo (Group A) or Bifidobacteria mixture (Group B) at baseline (T0) and after treatement (T1). Data are expressed as medians, IQR, and standard deviations
Fig. 2
QoL = practical problems item in patients treated with Placebo (Group A) or Bifidobacteria mixture (Group B) at baseline (T0) and after treatement (T1). Data are expressed as medians, IQR, and standard deviations
Similar articles
- Resveratrol plus carboxymethyl-β-glucan reduces nasal symptoms in children with pollen-induced allergic rhinitis.
Miraglia Del Giudice M, Maiello N, Capristo C, Alterio E, Capasso M, Perrone L, Ciprandi G. Miraglia Del Giudice M, et al. Curr Med Res Opin. 2014 Oct;30(10):1931-5. doi: 10.1185/03007995.2014.938731. Epub 2014 Jul 7. Curr Med Res Opin. 2014. PMID: 24983742 Clinical Trial. - A Mixture of 3 Bifidobacteria Decreases Abdominal Pain and Improves the Quality of Life in Children With Irritable Bowel Syndrome: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Crossover Trial.
Giannetti E, Maglione M, Alessandrella A, Strisciuglio C, De Giovanni D, Campanozzi A, Miele E, Staiano A. Giannetti E, et al. J Clin Gastroenterol. 2017 Jan;51(1):e5-e10. doi: 10.1097/MCG.0000000000000528. J Clin Gastroenterol. 2017. PMID: 27306945 Clinical Trial. - Effect of probiotic Bifidobacterium longum BB536 [corrected] in relieving clinical symptoms and modulating plasma cytokine levels of Japanese cedar pollinosis during the pollen season. A randomized double-blind, placebo-controlled trial.
Xiao JZ, Kondo S, Yanagisawa N, Takahashi N, Odamaki T, Iwabuchi N, Iwatsuki K, Kokubo S, Togashi H, Enomoto K, Enomoto T. Xiao JZ, et al. J Investig Allergol Clin Immunol. 2006;16(2):86-93. J Investig Allergol Clin Immunol. 2006. PMID: 16689181 Clinical Trial. - Desloratadine treatment for intermittent and persistent allergic rhinitis: a review.
Bachert C, van Cauwenberge P. Bachert C, et al. Clin Ther. 2007 Sep;29(9):1795-802. doi: 10.1016/j.clinthera.2007.09.009. Clin Ther. 2007. PMID: 18035184 Review. - Identification and management of undiagnosed and undertreated allergic rhinitis in adults and children.
Stewart MG. Stewart MG. Clin Exp Allergy. 2008 May;38(5):751-60. doi: 10.1111/j.1365-2222.2008.02937.x. Clin Exp Allergy. 2008. PMID: 18419620 Review.
Cited by
- Impact of Bifidobacterium longum Subspecies infantis on Pediatric Gut Health and Nutrition: Current Evidence and Future Directions.
Dargenio VN, Cristofori F, Brindicci VF, Schettini F, Dargenio C, Castellaneta SP, Iannone A, Francavilla R. Dargenio VN, et al. Nutrients. 2024 Oct 16;16(20):3510. doi: 10.3390/nu16203510. Nutrients. 2024. PMID: 39458503 Free PMC article. Review. - Efficacy of Bifidobacterium longum and Lactobacillus plantarum (NVP-1703) in Children With Allergic Rhinitis: A Randomized Controlled Trial.
Jeong K, Jang SW, Jeon SA, Seo HJ, Kang SH, Han SW, Suh DI, Lee S. Jeong K, et al. J Korean Med Sci. 2024 Oct 21;39(40):e266. doi: 10.3346/jkms.2024.39.e266. J Korean Med Sci. 2024. PMID: 39435516 Free PMC article. Clinical Trial. - Effects of probiotics on the prevention and treatment of children with allergic rhinitis: a meta-analysis of randomized controlled trials.
Luo X, Wang H, Liu H, Chen Y, Tian L, Ji Q, Xie D. Luo X, et al. Front Pediatr. 2024 Oct 3;12:1352879. doi: 10.3389/fped.2024.1352879. eCollection 2024. Front Pediatr. 2024. PMID: 39421038 Free PMC article. - Bacteria and Allergic Diseases.
Guryanova SV. Guryanova SV. Int J Mol Sci. 2024 Sep 25;25(19):10298. doi: 10.3390/ijms251910298. Int J Mol Sci. 2024. PMID: 39408628 Free PMC article. Review. - Probiotics in the New Era of Human Milk Oligosaccharides (HMOs): HMO Utilization and Beneficial Effects of Bifidobacterium longum subsp. infantis M-63 on Infant Health.
Wong CB, Huang H, Ning Y, Xiao J. Wong CB, et al. Microorganisms. 2024 May 17;12(5):1014. doi: 10.3390/microorganisms12051014. Microorganisms. 2024. PMID: 38792843 Free PMC article. Review.
References
- Dondi A, Tripodi S, Panetta V, Asero R, DiRienzo-Businco A, Bianchi A, et al. Italian Pediatric Allergy Network (I-PAN). Pollen-induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol. 2013;24:742–51. doi: 10.1111/pai.12136. - DOI - PubMed
- Global Initiative for Asthma. GINA guidelines. Global strategy for Asthma Management and Prevention. Available at: htpp://www.ginasthma.org/. Accessed July 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials