Active epithelial Hippo signaling in idiopathic pulmonary fibrosis - PubMed (original) (raw)
Active epithelial Hippo signaling in idiopathic pulmonary fibrosis
Jason J Gokey et al. JCI Insight. 2018.
Abstract
Hippo/YAP signaling plays pleiotropic roles in the regulation of cell proliferation and differentiation during organogenesis and tissue repair. Herein we demonstrate increased YAP activity in respiratory epithelial cells in lungs of patients with idiopathic pulmonary fibrosis (IPF), a common, lethal form of interstitial lung disease (ILD). Immunofluorescence staining in IPF epithelial cells demonstrated increased nuclear YAP and loss of MST1/2. Bioinformatic analyses of epithelial cell RNA profiles predicted increased activity of YAP and increased canonical mTOR/PI3K/AKT signaling in IPF. Phospho-S6 (p-S6) and p-PTEN were increased in IPF epithelial cells, consistent with activation of mTOR signaling. Expression of YAP (S127A), a constitutively active form of YAP, in human bronchial epithelial cells (HBEC3s) increased p-S6 and p-PI3K, cell proliferation and migration, processes that were inhibited by the YAP-TEAD inhibitor verteporfin. Activation of p-S6 was required for enhancing and stabilizing YAP, and the p-S6 inhibitor temsirolimus blocked nuclear YAP localization and suppressed expression of YAP target genes CTGF, AXL, and AJUBA (JUB). YAP and mTOR/p-S6 signaling pathways interact to induce cell proliferation and migration, and inhibit epithelial cell differentiation that may contribute to the pathogenesis of IPF.
Keywords: Cell Biology; Pulmonary surfactants; Pulmonology.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures
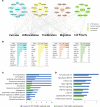
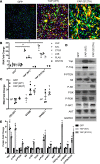
Figure 1. Prediction of signaling interactions in idiopathic pulmonary fibrosis (IPF) epithelial cells.
(A) Ingenuity pathway analysis of RNA sequencing data from CD326+/HTII-280+ sorted epithelial cells from healthy donors (n = 3) and IPF (n = 3) was used to predict intensive interactions among mTOR/PI3K/AKT, Hippo/YAP, and polarity signaling pathways. (B) Genes associated with each of the pathways significantly altered in IPF are shown. Each pathway is represented by a distinct color code: mTOR (blue), PI3K/AKT (yellow), Hippo (pink), and polarity (green). (C and D) Functional enrichment analysis predicted that genes induced in CD326+/HTII-280+ IPF epithelial cells (8) and in human airway epithelial cells (HAECs) expressing YAP (20) share (C) commonly activated bioprocesses and (D) signaling pathways including those affecting epithelial cell proliferation, migration, and cell size. The x axis represents the –log10-transformed enrichment P value.
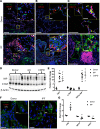
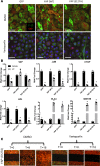
Figure 2. Hippo/YAP signaling in idiopathic pulmonary fibrosis (IPF).
(A–C) Representative immunofluorescence confocal microscopy of donor (n = 4) and IPF (n = 6) lung tissue was used to detect Hippo components. (A) AJUBA (white), ACTA2 (green), and ABCA3 (red). (B) YAP (green) and ABCA3 (red). (C) MST1/2 (red) and ABCA3 (green). White arrows point to cells coexpressing ABCA3 and nuclear YAP in IPF. Scale bars: 100 μm (overviews) and 10 μm (insets). (D) Representative immunoblots of YAP and phosphorylated YAP (p-YAP) from lung tissue lysates of IPF (n = 6), control (n = 4), and COPD (n = 3) patients are shown. (E) Western blots were normalized to β-actin. (F) Multiplexed proximity ligation fluorescence in situ hybridization (PLISH) staining of CTGF (red) and AXL (white) were costained using pan-cytokeratin (PanKRT) (green) in normal donor (n = 6) and IPF (n = 6) lung tissue. Scale bars: 25 μm and 2.5 μm (insets). (G) qPCR analysis of RNA from CD326+ epithelial cells isolated from peripheral lungs of healthy donor (n = 3) and IPF (n = 3) lung tissue was used to quantify SAV1, MST2, and JUB RNAs. ANOVA was used to assess Western blot quantification; Student’s t test was used for qPCR. *P < 0.05.
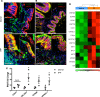
Figure 3. Vangl and Scribble in idiopathic pulmonary fibrosis (IPF) lung tissue.
(A and B) Representative immunofluorescence imaging of healthy donor (n = 4) and IPF (n = 6) lung tissue are shown. (A) Scribble (Scrib) (green) and Krt8 (red) or (B) Vangl (red) and Krt8 (green). Scale bars: 50 μm and 5 μm (insets). (C) CDH1, CELSR1, SCRIB, and VANGL1 RNAs were measured in CD326+ sorted epithelial cells from healthy donors (n = 3) and IPF (n = 3) lungs. (D) RNA sequencing analysis of polarity-associated genes of healthy donors (n = 3) and IPF (n = 3) demonstrating significant increases in epithelial cell polarity genes including WNT9A and WNT7A. *P < 0.05, calculated by Student’s t test. N.S., not significant.
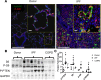
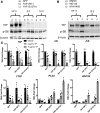
Figure 4. Increased mTOR signaling in idiopathic pulmonary fibrosis (IPF) respiratory epithelium.
(A) Representative immunofluorescence imaging of healthy donor (n = 3) and IPF (n = 3) lung for phosphorylated S6K (p-S6K) (red) with pan-cytokeratin (Pan-KRT) (green) is shown. Scale bars: 50 μm and 5 μm (insets). (B) Representative immunoblots were prepared from whole-lung lysates of donor (n = 4), COPD (n = 3), and IPF (n = 6) lung tissue for total S6, phosphorylated S6 (p-S6), and phosphorylated PTEN (p-PTEN). (C) Western blots were normalized to GAPDH. *P < 0.05, determined by ANOVA.
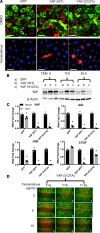
Figure 5. YAP (S127A) activates mTOR/PI3K/AKT.
(A) HBEC3s were transduced with lentiviruses expressing YAP (WT), YAP (S127A), or empty GFP vector. YAP (red) was identified by immunofluorescence. Images are representative of (n = 3) transductions. Scale bars: 10 μm. (B) qPCR analysis of cells 48 hours after transduction assessing genes regulated by YAP activity. (C) Analysis of polarity genes CELSR1, SCRIB, and VANGL1 in HBEC3s expressing YAP (WT) and YAP (MUT) for 48 hours. (D) Forty-eight hours after transduction, lysates were prepared and immunoblotted for YAP, p-YAP, p-PTEN, S6, p-S6, PI3K, p-PI3K, AKT, and p-AKT. (E) Western blot quantification normalized to GAPDH. *P < 0.05, assessed by ANOVA. HBEC3s, hTERT/CDK4–immortalized human bronchiolar epithelial cells.
Figure 6. Verteporfin inhibits YAP transcriptional activity and phosphorylation of S6 in ambient light.
HBEC3s were transduced with GFP, YAP (WT), or YAP (S127A) lentiviral vectors and treated with either vehicle (DMSO) or 0.25 μg/ml verteporfin for 48 hours (n = 3). (A) Immunofluorescence of p-S6 (green) and YAP (red) was assessed following verteporfin (0.25 μg/ml) or DMSO. Scale bars: 10 μm. (B) YAP, JUB, AXL, CTGF, WNT7B, and PLAU RNAs were quantified following 0.25 μg/ml verteporfin (VP) or vehicle exposed to ambient light (n = 3). Expression is normalized to DMSO controls. (C) Scratch assay of YAP (S127A)–transduced HBEC3s following DMSO or verteporfin treatment (0.25 μg/ml) in ambient light at T = 0, 8, and 16 hours of assay (n = 3). *P < 0.05, determined by ANOVA. HBEC3s, hTERT/CDK4–immortalized human bronchiolar epithelial cells.
Figure 7. Verteporfin reduces YAP transcriptional activity and phosphorylation of S6 in absence of light.
HBEC3s transduced with GFP, YAP (WT), or YAP (S127A) lentiviral vectors were treated with either vehicle (DMSO) or verteporfin (2.0 or 10.0 μg/ml) for 48 hours (n = 3). (A) Western blot for YAP and p-S6 following a verteporfin dose curve in complete darkness show reduced total YAP and p-S6. (B) Western blot analysis of YAP and p-S6 following verteporfin treatment for 48 hours with MHY1485 treatment for the final 24 hours shows increased YAP after mTOR activation. Addition of MG132 for the final 24 hours of verteporfin treatment protects YAP from degradation caused by verteporfin. (C) Yap, JUB, CTGF, AXL, PLAU, and WNT7B RNAs were assessed in experiments performed in complete darkness (n = 4 transfections). RNA expression is normalized to DMSO-treated cells. *P < 0.05, determined by ANOVA. HBEC3s, hTERT/CDK4–immortalized human bronchiolar epithelial cells.
Figure 8. Temsirolimus inhibits phosphorylation of S6, YAP transcriptional activity, and YAP-induced migration.
(A) Representative immunofluorescence microscopy was performed in HBEC3s transfected with GFP, YAP (WT), and YAP (S127A) and stained for p-S6 (green) and YAP (red) in the presence of temsirolimus (25 μg/ml) or DMSO (n = 5). Scale bars: 10 μm. (B) Western blot analysis of YAP following temsirolimus treatments shows reduced total YAP expression. (C) YAP, JUB, AXL, and CTGF RNAs were assessed after 48 hours (n = 3). RNA expression is normalized to DMSO-treated cells. (D) Time-lapse imaging of HBEC3s transduced with YAP (S127A) following treatment with temsirolimus at T = 0, 8, and 16 hours of a scratch assay (n = 3). *P < 0.05, assessed by ANOVA. HBEC3s, hTERT/CDK4–immortalized human bronchiolar epithelial cells.
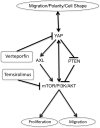
Figure 9. Proposed polarity/YAP/mTOR network regulating epithelial cell processes.
Cell polarity is regulated by and regulates YAP activity. YAP expression alters cell shape. YAP (S127A) expression inhibits PTEN, increases AXL, and activates mTOR with increased p-S6, p-S6K, and p-PI3K. Blocking mTOR activity with temsirolimus inhibits YAP-induced cell proliferation and migration, and also reduces nuclear YAP staining. Verteporfin inhibits YAP nuclear localization and transcriptional activity, while reduced p-S6 staining indicates reduced mTOR.
Similar articles
- Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung.
Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Lange AW, et al. J Mol Cell Biol. 2015 Feb;7(1):35-47. doi: 10.1093/jmcb/mju046. Epub 2014 Dec 5. J Mol Cell Biol. 2015. PMID: 25480985 Free PMC article. - PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis.
Xu W, Yang Z, Xie C, Zhu Y, Shu X, Zhang Z, Li N, Chai N, Zhang S, Wu K, Nie Y, Lu N. Xu W, et al. J Exp Clin Cancer Res. 2018 Aug 22;37(1):198. doi: 10.1186/s13046-018-0795-2. J Exp Clin Cancer Res. 2018. PMID: 30134988 Free PMC article. - A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth.
Lin C, Yao E, Chuang PT. Lin C, et al. Dev Biol. 2015 Jul 1;403(1):101-13. doi: 10.1016/j.ydbio.2015.04.014. Epub 2015 Apr 24. Dev Biol. 2015. PMID: 25912685 Free PMC article. - YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis.
Papavassiliou KA, Sofianidi AA, Spiliopoulos FG, Gogou VA, Gargalionis AN, Papavassiliou AG. Papavassiliou KA, et al. Cells. 2024 Sep 10;13(18):1519. doi: 10.3390/cells13181519. Cells. 2024. PMID: 39329703 Free PMC article. Review. - Targeting YAP and Hippo signaling pathway in liver cancer.
Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Liu AM, et al. Expert Opin Ther Targets. 2010 Aug;14(8):855-68. doi: 10.1517/14728222.2010.499361. Expert Opin Ther Targets. 2010. PMID: 20545481 Review.
Cited by
- Inhibiting endothelial cell Mst1 attenuates acute lung injury in mice.
Guo ZF, Tongmuang N, Li C, Zhang C, Hu L, Capreri D, Zuo MX, Summer R, Sun J. Guo ZF, et al. JCI Insight. 2024 Sep 10;9(17):e178208. doi: 10.1172/jci.insight.178208. JCI Insight. 2024. PMID: 39253972 Free PMC article. - Cutting the ovarian surface improves the responsiveness to exogenous hormonal treatment in aged mice.
Umehara T, Urabe N, Obata T, Yamaguchi T, Tanaka A, Shimada M. Umehara T, et al. Reprod Med Biol. 2020 Aug 26;19(4):415-424. doi: 10.1002/rmb2.12345. eCollection 2020 Oct. Reprod Med Biol. 2020. PMID: 33071644 Free PMC article. - A new, easily generated mouse model of diabetic kidney fibrosis.
He X, Zhang T, Tolosa M, Goru SK, Chen X, Misra PS, Robinson LA, Yuen DA. He X, et al. Sci Rep. 2019 Aug 29;9(1):12549. doi: 10.1038/s41598-019-49012-4. Sci Rep. 2019. PMID: 31467329 Free PMC article. - Identification of biomarkers by machine learning classifiers to assist diagnose rheumatoid arthritis-associated interstitial lung disease.
Qin Y, Wang Y, Meng F, Feng M, Zhao X, Gao C, Luo J. Qin Y, et al. Arthritis Res Ther. 2022 May 19;24(1):115. doi: 10.1186/s13075-022-02800-2. Arthritis Res Ther. 2022. PMID: 35590341 Free PMC article. - The Role of Hippo/YAP Signaling in Alveolar Repair and Pulmonary Fibrosis.
Gokey JJ, Patel SD, Kropski JA. Gokey JJ, et al. Front Med (Lausanne). 2021 Oct 4;8:752316. doi: 10.3389/fmed.2021.752316. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34671628 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R56 HL123969/HL/NHLBI NIH HHS/United States
- T32 HL007752/HL/NHLBI NIH HHS/United States
- U01 HL110964/HL/NHLBI NIH HHS/United States
- R01 HL131661/HL/NHLBI NIH HHS/United States
- U01 HL122642/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous