Human Enteric α-Defensin 5 Promotes Shigella Infection by Enhancing Bacterial Adhesion and Invasion - PubMed (original) (raw)
. 2018 Jun 19;48(6):1233-1244.e6.
doi: 10.1016/j.immuni.2018.04.014. Epub 2018 May 29.
Chongbing Liao 2, Bing Zhang 3, W David Tolbert 4, Wangxiao He 1, Zhijun Dai 5, Wei Zhang 3, Weirong Yuan 4, Marzena Pazgier 4, Jiankang Liu 3, Jun Yu 6, Philippe J Sansonetti 7, Charles L Bevins 8, Yongping Shao 9, Wuyuan Lu 10
Affiliations
- PMID: 29858013
- PMCID: PMC6051418
- DOI: 10.1016/j.immuni.2018.04.014
Human Enteric α-Defensin 5 Promotes Shigella Infection by Enhancing Bacterial Adhesion and Invasion
Dan Xu et al. Immunity. 2018.
Abstract
Shigella is a Gram-negative bacterium that causes bacillary dysentery worldwide. It invades the intestinal epithelium to elicit intense inflammation and tissue damage, yet the underlying mechanisms of its host selectivity and low infectious inoculum remain perplexing. Here, we report that Shigella co-opts human α-defensin 5 (HD5), a host defense peptide important for intestinal homeostasis and innate immunity, to enhance its adhesion to and invasion of mucosal tissues. HD5 promoted Shigella infection in vitro in a structure-dependent manner. Shigella, commonly devoid of an effective host-adhesion apparatus, preferentially targeted HD5 to augment its ability to colonize the intestinal epithelium through interactions with multiple bacterial membrane proteins. HD5 exacerbated infectivity and Shigella-induced pathology in a culture of human colorectal tissues and three animal models. Our findings illuminate how Shigella exploits innate immunity by turning HD5 into a virulence factor for infection, unveiling a mechanism of action for this highly proficient human pathogen.
Keywords: Shigella; antimicrobial peptide; bacterial adhesion; defensin; enteropathogenic bacteria; epithelial integrity; host-microbe interaction; innate immunity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures
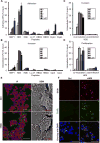
Fig. 1. HD5 promotes Shigella infection of epithelial cells in vitro
(A, B) The effects of eight antimicrobial peptides at sub-lethal concentrations on Shigella flexneri Sf301 adhesion (A) to and invasion (B) of HeLa cells during bacterial infection. (C, D) The effects of HD5 treatment on Sf301 invasion (C) and proliferation (D) when added during initial infection (co-incubation) or after invasion (post-infection). Adhesion, invasion and proliferation assays were performed as described in Methods. Experimental details are illustrated in Fig. S2. Data are shown as mean ± SD of at least three independent experiments. Statistical significance was calculated (for peptide-treated samples compared to vehicle controls (0 μM)) using a one-way ANOVA (Dunnett’s multiple comparison Test), and p values are as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. (E) Fluorescence microscopy (left panels) and scanning electron microscopy (right panels) analysis of Sf301 adhesion to HeLa cells in the absence (control) or presence of 4 μM HD5 (MOI=50:1). GFP-expressing bacteria are green, β1 integrin is red, and nuclei are blue (DAPI). For fluorescent images, the scale bars represent 50 m; for SEM images, the bars represent 10 μm. (F) Confocal microscopy images of HeLa cells infected for 60 min with mCherry-labeled WT Sf301 harboring a GFP-expressing reporter plasmid (Campbell-Valois et al., 2014) in presence or absence of 4 μM HD5. HeLa cells are counterstained with DAPI, and GFP expression is induced upon activation of type 3 secretion system triggered by bacterial cell contact with the host. Note that red and green overlay gives rise to yellow. The scale bars represent 50 μm.
Fig. 2. HD5 promotes Shigella infection in vivo
(A) Sereny test of Shigella infection using guinea pigs. Hartley guinea pigs (6–8 weeks of age) were inoculated with 106 CFU/eye of mid-log phase Sf301 either in the absence (n=15) and presence of HD5 (either 4 μM (n=14) or 8 μM (n=14)). A control group (n=6) was inoculated with HD5 alone (8 μM). Animals were observed and scored for the development of conjunctivitis over 7 consecutive days. Eye pathology was independently scored by three individuals (blinded to treatment group) on a scale of 0–3, grade 0 (no disease or mild irritation), grade 1 (mild conjunctivitis or late development and/or rapid clearing of symptoms), grade 2 (keratoconjunctivitis without purulence), and grade 3 (fully developed keratoconjunctivitis with purulence) as indicated in Fig. S3A. The data are representative of three independent experiments. Each point represents a single animal. Statistical significance compared with inoculation of 106 CFU in the absence of HD5 was determined using a Mann-Whitney test, *p < 0.05, **p < 0.01. Please also see Fig. S3A for daily scoring. (B–H) Colon infection model with guinea pigs. Hartley guinea pigs (6–8 weeks of age) were inoculated intrarectally by 1×108 CFU of HD5-treated (8 μM), 1×108 CFU of mock-treated, 1×109 CFU of mock-treated GFP-expressing Sf301 or medium alone, with 20 animals in each of the three treatment groups and 8 in the negative control group. Animals were monitored for 48h, and the distal 10 cm of colon tissue from groups of euthanized animals was harvested for analysis at 4, 8, 24 and 48 h post-challenge. (B) Confocal microscopy images of representative colon sections at 4 h post-challenge, counterstained with DAPI. The scale bars represent 50 μm. Please also see Fig. S3B for images at 8h and 24h post-challenge. The ten most bacteria-enriched fields from each experimental group were analyzed at 4 h (n=3), 8 h (n=3), 24 h (n=5) and 48 h (n=5) post-challenge, followed by automated enumeration of individual bacteria (C). Results are representative of three independent experiments and are shown as mean ± SD. Statistical significance in comparison with 1×108 CFU of mock-treated group at each time point was calculated using a one-way ANOVA (Dunnett’s multiple comparison Test), and p values are as follows: *p < 0.05, **p < 0.01. (D) SEM analysis of bacterial infection of the colonic mucosa at 2h post-challenge. The scale bars represent 5 μm. (E) Histopathology analysis of representative colon sections at 24 h and 48 h post-challenge by HE staining. The scale bars represent 100 μm. Colon histopathology scores at 24 h (F) or 48 h (G) (n=5, each) were assigned as follows: 0, intact colonic architecture, no acute inflammation or epithelial injury; 1, focal minimal acute inflammation; 2, focal mild acute inflammation; 3, severe acute inflammation with multiple crypt abscesses and/or focal ulceration; 4, severe acute inflammation, multiple crypt abscesses, epithelial loss, and extensive ulceration. Results are representative of three independent experiments. Indicated are mean ± SEM. Each point represents a single animal. Statistical significance in comparison with 1×108 CFU of mock-treated group was determined using a Mann-Whitney test, *p < 0.05, **p < 0.01. (H) Core body temperature of animals 48 h post-challenge. Results are representative of three independent experiments. Indicated are mean ± SD. Each point represents a single animal (n=9). Statistical significance between indicated groups was determined using a one-way ANOVA (Tukey’s multiple comparison Test), *p < 0.05, **p < 0.01. (I–J) Ileum and colon infection model in mice. For the colon lops (I), a small abdominal incision was made in fasted, anesthetized mice and two separate loops of colon were isolated by suture (2–3 cm in length). For each animal, one isolated colonic loop was instilled with 1×107 CFU of HD5-treated Sf301, and the second loop with mock-treated Sf301 (both in 100 μl medium). For the ileal loop model (J), the same experimental approach was employed except that the two sutured loops were with the distal ileum. Two-hours post inoculation, mice were euthanized, and loops were harvested for quantitative fluorescence imaging of Shigella infection. The scale bars represent 50 μm. Results are shown as mean ± SD. Statistical significance in comparison with group challenged with 1×107 CFU of mock-treated bacteria was calculated using a t-test, and “*” indicates p < 0.05.
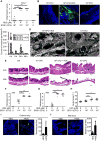
Fig. 3. HD5 promotes Shigella infection ex vivo
(A) SEM analysis of the bacterial adhesion (red arrows) to human colonic mucosa 30 min after inoculation of 106 CFU Sf301 in the absence (control) and presence of HD5 (8 μM). The scale bars represent 10 μm. (B) SEM analysis of bacterial invasion of human colonic tissue at 2 h post-inoculation in presence of 8 μM HD5. Clustered bacteria are indicated by red circles in the upper panel (the scale bar represents 100 μm), and individual bacteria by red arrows in the lower panel (the scale bar represents 5 μm). (C, D) Analysis of histopathology of human colorectal explants 2 h after the inoculation either with or without Sf301 in either the absence or presence of HD5 (8 μM). Untreated (control) and HD5-treated specimens are included for comparison. Experimental details are described in Methods. Colon histopathology scores (C) were assigned as described above. Results are representative of three independent experiments. Indicated are mean ± SEM. Each point represents a colon sample. Statistical significance was determined using a Mann-Whitney test, *p < 0.05. Representative images of HE staining (the scale bars represent 100 μm) and SEM analysis (the scale bars represent 500 μm) of colonic mucosa at 2h post-challenge are shown in D. Please also see Fig. S3C for experimental procedure.
Fig. 4. (A–C) HD5 potentiates destruction of the epithelium by Shigella
(A) Trans-epithelial electrical resistance (TEER) analysis of epithelial integrity of the monolayer of polarized Caco-2 cells infected with Sf301 in either the absence or presence of HD5 (8 μM). Percent TEER values were normalized against values of each treatment group at time 0. Data are mean ± SD from at least three independent experiments. Statistical significance was determined using a two-way ANOVA, **, p < 0.01; ***, p < 0.001. (B) Intracellular CFU of the polarized Caco-2 monolayer 6 h post-inoculation of Sf301 in either the absence or presence of HD5 (8 μM). Data are mean ± SD from at least three independent experiments. Statistical significance was determined using a t test ****, p < 0.0001. (C) Disruption of the tight junction (as shown by immunofluorescence) and impairment of the integrity (as shown by SEM) of the epithelium. Polarized Caco-2 cells grown in transwell inserts were infected with Sf301 in either the absence or presence of HD5 (8 μM) for 6 h, followed by immunostaining with antibody against the tight junction marker ZO-1 (green). Nuclei are stained with DAPI (blue). The scale bars represent 50 μm. Invading bacteria in the polarized Caco-2 monolayer are indicated by red arrows in the SEM image. The scale bars represent 2 μm. (D, E) Effects of concentrated ileal fluid aspirates on Shigella Sf301 adhesion (D) and invasion (E) in comparison with DMEM either without or with HD5 (4 μM). Anti-HD5 antiserum was added to ileal fluids and HD5-containing DMEM at a dilution titer of 1:100 to examine its neutralizing activity against Shigella infection promoted by endogenous HD5. Data are shown as mean ± SD of at least three independent experiments. Statistical significance between indicated groups was determined using a one-way ANOVA (Tukey’s multiple comparison test), and p values are as follows: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
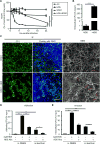
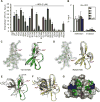
Fig. 5. Structural determinants of HD5 function
(A, B) Activities of native and alanine mutants of HD5 (A) and Abu-HD5 (B) on Shigella adhesion and invasion (for one hour). The adhesion and invasion assays were as in Fig. 1A, and data are expressed as the number of intracellular (A, B) and adhering (B) bacteria in HeLa cells relative to the input. Data are shown as mean ± SD of at least three independent experiments. Statistical significance in comparison with wildtype HD5 in A and in comparison with solvent control group in B was determined using a one-way ANOVA (Dunnett’s multiple comparison Test), and p values are as follows: *p < 0.05, **p < 0.01, ***p < 0.001. (C, D) The 2Fo-Fc electron density map contoured at 1.0σ of molecule A of Y27A-HD5 (C) and R28A-HD5 crystal (D) and the superimposition of defensin molecules present in the asymmetric units of analogs’ crystals with the wildtype HD5 monomers (shown in grey, from PDB code: 1ZMP (Szyk et al., 2006)). Side chains of cysteines forming disulfide bridges and mutated residues are shown as sticks. Structural analysis of Y27A-HD5 and R28A-HD5 analogs confirms that both mutant monomers assume the same fold as the wildtype HD5 monomer with no major changes to the overall structure and the network of three disulfide bridges. When superimposed, the root-mean-square deviations (RMSD) between 128 equivalent main chain atoms of wildtype HD5 and Y27A-HD5 and R28A-HD5 are in the range of 0.91–1.33 Å and 0.35–0.95 Å, respectively. (E, F) Crystal structures of the Y27A-HD5 monomer (E, green) and the R28A-HD5 dimer (F, yellow) superimposed on the wildtype HD5 dimer in grey (PDB code: 1ZMP). Mutated residues and alanine substitutions are shown as spheres. (G) Key functional residues of HD5 forming putative binding surfaces for interactions with bacterial and host proteins. Positively charged Arg28 residues are colored in blue, and hydrophobic residues Leu16, Val19, and Leu26 in green. Shades of green depict differences in activity with residues in light green being less important than those in dark green. Important residues not depicted in this view are Tyr27 and Leu29.
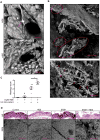
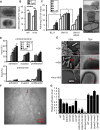
Fig. 6. Bacterial determinants of HD5-promoted Shigella infection
(A) TEM analysis of fimbriae expression in Sf301. The Fim cassette from E. coli JM103 was expressed from arabinose-inducible pBAD vector in Sf301. The scale bars represent 1 μm. (B) Influence of fimbriae-expression on the adhesion and invasion of Sf301 in the absence or presence of HD5. Data are shown as mean ± SD of at least three independent experiments. Statistical significance was determined using a two-way ANOVA, and p values are as follows: *p < 0.05, **p < 0.01 and ***p < 0.001. (C) Host cell-adhesion activity of fimbriated and non-fimbriated E. coli strains in the absence or presence of HD5. Adhesion assays to HeLa cells were carried out with fimbriated E. coli (JM103), its fimbriaedeficient mutant (JM103 Δfim) and non-fimbriated E. coli (BL21). TEM images of these strains are shown on the right panel. The scale bars represent 500 nm. Statistical significance between indicated groups was determined using a t test, and p values are as follows: *p < 0.05, **p < 0.01 and ***p < 0.001. (D) Either Sf301 bacteria, or the HeLa cells, were pre-treated with HD5 at the indicated concentrations for 30 min, washed once with DMEM, and the adhesion and invasion assays were performed. Data are shown as mean ± SD of at least three independent experiments. Statistical significance compared with solvent control (0 μM) group at each time point was determined using a one-way ANOVA (Dunnett’s multiple comparison test), and p values are as follows: *p < 0.05, **p < 0.01, and ***p < 0.001. (E) SEM and TEM analysis of Sf301 treated with HD5 or its linear analogue (Abu-HD5). The scale bars represent 2 μM for SEM and 500 nm for TEM. (F) Immunogold-TEM analysis of HD5 localization in Sf301-HeLa interaction in presence of HD5. HD5 was labeled by ~12 nm colloidal gold particles and TEM were performed as described in Methods. B, bacterium; C, Cell. The scale bars represent 500 nm. Please also see Fig. S5B for more Immunogold-TEM images showing HD5 bridging single bacterium to host and HD5 clustering multiple bacteria. (G) Relative adhesion ability of different Sf301 mutants in the presence of 4 μM HD5. spa33, icsA, ompA, ompC, ompF genes and some combinations of two were ablated as described in Methods. Shigella strains were transformed with pBAD plasmids carrying the OmpC coding sequence and induced with 10 mM L-arabinose for OmpC expression. Please also see Fig. S6F for SDS-PAGE analysis of the genetic ablations and recompletions. Adhesion assays were performed as in Fig. 1A. Data are normalized to the input and shown as the percentage of the adherent bacteria of wild-type Sf301 in the presence of 4 μM HD5. Data are shown as mean ± SD of at least three independent experiments. Statistical significance compared with wild type was determined using a one-way ANOVA (Dunnett’s multiple comparison test), and p values are as follows: *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Comment in
- Defens-IN! Human α-Defensin 5 Acts as an Unwitting Double Agent to Promote Shigella Infection.
Murphy AG, Maloy KJ. Murphy AG, et al. Immunity. 2018 Jun 19;48(6):1070-1072. doi: 10.1016/j.immuni.2018.05.015. Immunity. 2018. PMID: 29924970
Similar articles
- Human Enteric Defensin 5 Promotes Shigella Infection of Macrophages.
Xu D, Liao C, Xiao J, Fang K, Zhang W, Yuan W, Lu W. Xu D, et al. Infect Immun. 2019 Dec 17;88(1):e00769-19. doi: 10.1128/IAI.00769-19. Print 2019 Dec 17. Infect Immun. 2019. PMID: 31611271 Free PMC article. - Critical determinants of human neutrophil peptide 1 for enhancing host epithelial adhesion of Shigella flexneri.
Liao C, Fang K, Xiao J, Zhang W, Zhang B, Yuan W, Lu W, Xu D. Liao C, et al. Cell Microbiol. 2019 Oct;21(10):e13069. doi: 10.1111/cmi.13069. Epub 2019 Jul 5. Cell Microbiol. 2019. PMID: 31218775 - Shigella infection of intestinal epithelium and circumvention of the host innate defense system.
Ashida H, Ogawa M, Mimuro H, Sasakawa C. Ashida H, et al. Curr Top Microbiol Immunol. 2009;337:231-55. doi: 10.1007/978-3-642-01846-6_8. Curr Top Microbiol Immunol. 2009. PMID: 19812985 Review. - Defens-IN! Human α-Defensin 5 Acts as an Unwitting Double Agent to Promote Shigella Infection.
Murphy AG, Maloy KJ. Murphy AG, et al. Immunity. 2018 Jun 19;48(6):1070-1072. doi: 10.1016/j.immuni.2018.05.015. Immunity. 2018. PMID: 29924970 - Shigella host: Pathogen interactions: Keeping bacteria in the loop.
Liu G, Pilla G, Tang CM. Liu G, et al. Cell Microbiol. 2019 Nov;21(11):e13062. doi: 10.1111/cmi.13062. Epub 2019 Jul 2. Cell Microbiol. 2019. PMID: 31134722 Review.
Cited by
- An Oral Inoculation Infant Rabbit Model for Shigella Infection.
Kuehl CJ, D'Gama JD, Warr AR, Waldor MK. Kuehl CJ, et al. mBio. 2020 Jan 21;11(1):e03105-19. doi: 10.1128/mBio.03105-19. mBio. 2020. PMID: 31964739 Free PMC article. - Mouse α-Defensins: Structural and Functional Analysis of the 17 Cryptdin Isoforms Identified from a Single Jejunal Crypt.
Wang Q, Yang Y, Luo G, Zhou Y, Tolbert WD, Pazgier M, Liao C, Lu W. Wang Q, et al. Infect Immun. 2023 Jan 24;91(1):e0036122. doi: 10.1128/iai.00361-22. Epub 2022 Dec 6. Infect Immun. 2023. PMID: 36472443 Free PMC article. - Inhibitory Activity of a Scorpion Defensin BmKDfsin3 against Hepatitis C Virus.
Cheng Y, Sun F, Li S, Gao M, Wang L, Sarhan M, Abdel-Rahman MA, Li W, Kwok HF, Wu Y, Cao Z. Cheng Y, et al. Antibiotics (Basel). 2020 Jan 17;9(1):33. doi: 10.3390/antibiotics9010033. Antibiotics (Basel). 2020. PMID: 31963532 Free PMC article. - Understanding the Dynamics of Human Defensin Antimicrobial Peptides: Pathogen Resistance and Commensal Induction.
Kumaresan V, Kamaraj Y, Subramaniyan S, Punamalai G. Kumaresan V, et al. Appl Biochem Biotechnol. 2024 Oct;196(10):6993-7024. doi: 10.1007/s12010-024-04893-8. Epub 2024 Mar 13. Appl Biochem Biotechnol. 2024. PMID: 38478321 Review. - Animal Models of Type III Secretion System-Mediated Pathogenesis.
Hotinger JA, May AE. Hotinger JA, et al. Pathogens. 2019 Nov 22;8(4):257. doi: 10.3390/pathogens8040257. Pathogens. 2019. PMID: 31766664 Free PMC article. Review.
References
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous