The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma - PubMed (original) (raw)
Review
The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma
Jacopo Baglieri et al. Int J Mol Sci. 2019.
Abstract
Hepatocellular carcinoma (HCC) is one of the most aggressive types of cancer and lacks effective therapeutic approaches. Most HCC develops in the setting of chronic liver injury, hepatic inflammation, and fibrosis. Hepatic stellate cells (HSCs) and cancer-associated fibroblasts (CAFs) are key players in liver fibrogenesis and hepatocarcinogenesis, respectively. CAFs, which probably derive from HSCs, activate into extracellular matrix (ECM)-producing myofibroblasts and crosstalk with cancer cells to affect tumor growth and invasion. In this review, we describe the different components which form the HCC premalignant microenvironment (PME) and the tumor microenvironment (TME), focusing on the liver fibrosis process and the biology of CAFs. We will describe the CAF-dependent mechanisms which have been suggested to promote hepatocarcinogenesis, such as the alteration of ECM, CAF-dependent production of cytokines and angiogenic factors, CAF-dependent reduction of immuno-surveillance, and CAF-dependent promotion of epithelial-mesenchymal transition (EMT). New knowledge of the fibrosis process and the role of CAFs in HCC may pave the way for new therapeutic strategies for liver cancer.
Keywords: cancer-associated fibroblasts (CAFs); fibrosis; hepatic stellate cells (HSCs); hepatocellular carcinoma (HCC); tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript.
Figures
Figure 1
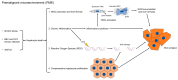
Mechanisms which promote HCC formation in PME. In PME, chronic liver injury causes hepatocyte death, which triggers inflammation, the activation of hepatic stellate cells into ECM-producing myofibroblasts, compensatory hepatocyte proliferation, the release of reactive oxygen species (ROS), and DNA damage. Continuous cycles of this destructive–regenerative process, which precedes tumor formation, are proposed to give rise to replication-related mutations in hepatocytes and eventually HCC. The links between the different mechanisms are indicated here.
Figure 2
TME in HCC. The figure outlines the different components of the TME and the CAF-dependent mechanisms of hepatocarcinogenesis. (See text for details).
Similar articles
- Inducible factors for cancer-associated fibroblasts in liver cancer versus myofibroblasts in inflammatory liver disease.
Okabe H, Hayashi H, Nakagawa S, Imai K, Nitta H, Arima K, Hashimoto D, Chikamoto A, Ishiko T, Beppu T, Baba H. Okabe H, et al. Histol Histopathol. 2016 Feb;31(2):141-8. doi: 10.14670/HH-11-668. Epub 2015 Sep 23. Histol Histopathol. 2016. PMID: 26398776 Review. - Cancer-associated fibroblasts in hepatocellular carcinoma.
Kubo N, Araki K, Kuwano H, Shirabe K. Kubo N, et al. World J Gastroenterol. 2016 Aug 14;22(30):6841-50. doi: 10.3748/wjg.v22.i30.6841. World J Gastroenterol. 2016. PMID: 27570421 Free PMC article. Review. - Components of the Hepatocellular Carcinoma Microenvironment and Their Role in Tumor Progression.
Novikova MV, Khromova NV, Kopnin PB. Novikova MV, et al. Biochemistry (Mosc). 2017 Aug;82(8):861-873. doi: 10.1134/S0006297917080016. Biochemistry (Mosc). 2017. PMID: 28941454 Review. - Hepatic Stellate Cell-Macrophage Crosstalk in Liver Fibrosis and Carcinogenesis.
Matsuda M, Seki E. Matsuda M, et al. Semin Liver Dis. 2020 Aug;40(3):307-320. doi: 10.1055/s-0040-1708876. Epub 2020 Apr 2. Semin Liver Dis. 2020. PMID: 32242330 Free PMC article. Review. - Hepatic stellate cells and extracellular matrix in hepatocellular carcinoma: more complicated than ever.
Carloni V, Luong TV, Rombouts K. Carloni V, et al. Liver Int. 2014 Jul;34(6):834-43. doi: 10.1111/liv.12465. Epub 2014 Feb 12. Liver Int. 2014. PMID: 24397349 Review.
Cited by
- Role of Fibroblast Growth Factors in the Crosstalk of Hepatic Stellate Cells and Uveal Melanoma Cells in the Liver Metastatic Niche.
Seitz T, John N, Sommer J, Dietrich P, Thasler WE, Hartmann A, Evert K, Lang SA, Bosserhoff A, Hellerbrand C. Seitz T, et al. Int J Mol Sci. 2022 Sep 29;23(19):11524. doi: 10.3390/ijms231911524. Int J Mol Sci. 2022. PMID: 36232829 Free PMC article. - Prediction of CAF-related genes in immunotherapy and drug sensitivity in hepatocellular carcinoma: a multi-database analysis.
Yao Y, Yang K, Wang Q, Zhu Z, Li S, Li B, Feng B, Tang C. Yao Y, et al. Genes Immun. 2024 Feb;25(1):55-65. doi: 10.1038/s41435-024-00252-z. Epub 2024 Jan 17. Genes Immun. 2024. PMID: 38233508 Free PMC article. - MyD88 in myofibroblasts enhances nonalcoholic fatty liver disease-related hepatocarcinogenesis via promoting macrophage M2 polarization.
Liu Y, Chen H, Yan X, Zhang J, Deng Z, Huang M, Gu J, Zhang J. Liu Y, et al. Cell Commun Signal. 2024 Jan 30;22(1):86. doi: 10.1186/s12964-024-01489-x. Cell Commun Signal. 2024. PMID: 38291436 Free PMC article. - Heterogeneity of cancer-associated fibroblasts and roles in the progression, prognosis, and therapy of hepatocellular carcinoma.
Yin Z, Dong C, Jiang K, Xu Z, Li R, Guo K, Shao S, Wang L. Yin Z, et al. J Hematol Oncol. 2019 Sep 23;12(1):101. doi: 10.1186/s13045-019-0782-x. J Hematol Oncol. 2019. PMID: 31547836 Free PMC article. Review. - Non-Parenchymal Cells and the Extracellular Matrix in Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease.
van Son KC, Verschuren L, Hanemaaijer R, Reeves H, Takkenberg RB, Drenth JPH, Tushuizen ME, Holleboom AG. van Son KC, et al. Cancers (Basel). 2023 Feb 18;15(4):1308. doi: 10.3390/cancers15041308. Cancers (Basel). 2023. PMID: 36831649 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- P42 ES010337/ES/NIEHS NIH HHS/United States
- R01 DK111866/DK/NIDDK NIH HHS/United States
- P50 AA011999/AA/NIAAA NIH HHS/United States
- R01 DK101737/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials