Gamma Radiation-Induced Disruption of Cellular Junctions in HUVECs Is Mediated through Affecting MAPK/NF- κ B Inflammatory Pathways - PubMed (original) (raw)
Gamma Radiation-Induced Disruption of Cellular Junctions in HUVECs Is Mediated through Affecting MAPK/NF- κ B Inflammatory Pathways
H Wang et al. Oxid Med Cell Longev. 2019.
Abstract
Ionizing radiation-induced cardiovascular diseases (CVDs) have been well documented. However, the mechanisms of CVD genesis are still not fully understood. In this study, human umbilical vein endothelial cells (HUVECs) were exposed to gamma irradiation at different doses ranging from 0.2 Gy to 5 Gy. Cell viability, migration ability, permeability, oxidative and nitrosative stresses, inflammation, and nuclear factor kappa-light-chain-enhancer of activated B cell (NF-_κ_B) pathway activation were evaluated postirradiation. It was found that gamma irradiation at doses ranging from 0.5 Gy to 5 Gy inhibited the migration ability of HUVECs without any significant effects on cell viability at 6 h and 24 h postirradiation. The decreased transendothelial electrical resistance (TEER), increased permeability, and disruption of cellular junctions were observed in HUVECs after gamma irradiation accompanied by the lower levels of junction-related proteins such as ZO-1, occludin, vascular endothelial- (VE-) cadherin, and connexin 40. The enhanced oxidative and nitrosative stresses, e.g., ROS and NO2 - levels and inflammatory cytokines IL-6 and TNF-α were demonstrated in HUVECs after gamma irradiation. Western blot results showed that protein levels of mitogen-activated protein kinase (MAPK) pathway molecules p38, p53, p21, and p27 increased after gamma irradiation, which further induced the activation of the NF-_κ_B pathway. BAY 11-7085, an inhibitor of NF-_κ_B activation, was demonstrated to partially block the effects of gamma radiation in HUVECs examined by TEER and FITC-dextran permeability assay. We therefore concluded that the gamma irradiation-induced disruption of cellular junctions in HUVECs was through the inflammatory MAPK/NF-_κ_B signaling pathway.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Figure 1
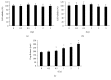
Cell viability and migration ability in HUVECs after gamma irradiation of different doses. Gamma irradiation with doses ranging from 0.2 Gy to 5 Gy does not induce HUVEC viability changes when measured at 6 h (a) and 24 h (b) postirradiation by the MTT method. However, it increased the gap distance when HUVECs were irradiated with radiation doses ranging from 0.5 to 5 Gy (c). The cell viability of the treatment groups is expressed as the percentage of the control; cell migration is measured by gap closure test. ∗A value of p < 0.05 is taken as statistical significance.
Figure 2
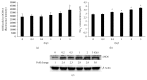
Cellular junction examination in HUVECs after exposure to gamma irradiation. Immunofluorescence examination indicates the damage of the cellular barrier 24 h after irradiation with 5 Gy (the impaired and disconnected junctions between two adjoined cells pointed by arrows in the lower panels in (a)) (×400). Scale bar: 50 _μ_m. While the gene expressions of junction-related molecules such as ZO-1, occludin, VE-cadherin, and connexin 40 do not change significantly (b), there are obvious reductions of protein expressions of ZO-1, occludin, VE-cadherin, and connexin 40 (c).
Figure 3
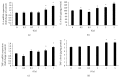
Gamma irradiation induces oxidative and nitrosative stresses in HUVECs. The intracellular ROS is increased significantly 6 h after irradiation with 5 Gy (a). NO2− concentration is increased significantly 6 h after irradiation with doses from 0.5 to 5 Gy (b). Western blot shows increased eNOS after irradiation with doses from 0.2 to 5 Gy (c). ∗A value of p < 0.05 is taken as statistical significance.
Figure 4
Gamma irradiation induces gene and protein expressions of cytokines. Irradiation with 2 or 5 Gy induces upregulation of the IL-6 gene 6 h after irradiation (a), but upregulation of the IL-6 protein is induced by irradiation with doses ranging from 0.2 to 5 Gy (b). Both gene (c) and protein (d) expressions of TNF-α are upregulated after irradiation with 2 or 5 Gy. ∗A value of p < 0.05 is taken as statistical significance.
Figure 5
Gamma irradiation activates MAPK/NF-_κ_B pathways. (a) The activation of MAPK pathway molecules p-p38, p38, p53, p21, and p27 in HUVECs after exposure to gamma irradiation was measured by western blot. (b) Protein levels of p-p65 and p65 were examined by western blot. (c) NF-_κ_B DNA-binding activity by TransAM™ NF-_κ_B Transcription Factor Assay kit. The absorbance at 450 nm was read on a microplate reader. Values were expressed as mean ± S.E.M. One-way analysis of variance (ANOVA) was used to determine statistical significance between groups followed by Tukey's post hoc test. ∗A value of p < 0.05 was taken as statistical significance.
Figure 6
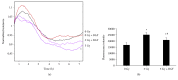
BAY 11-7085 treatment on the barrier function examined by ECIS and FITC-dextran permeability assay. Irradiation with 5 Gy significantly reduces the resistance in HUVECs when compared to the nonirradiated (0 Gy) group (a). The treatment with BAY 11-7085 at 2.5 μ_M partially blocked the decreased resistance in HUVECs caused by 5 Gy gamma irradiation, while the treatment alone did not have any significant effects in HUVECs without gamma radiation exposure (a). The fluoresce intensity of FITC-labelled dextrans (4 kDa) was significantly higher after 5 Gy gamma irradiation, and the treatment of BAY 11-7085 partially inhibited this increase (b). ∗_p < 0.05 vs. 0 Gy; #p < 0.05 vs. 5 Gy.
Similar articles
- Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways.
Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K, Sun H. Xia F, et al. J Atheroscler Thromb. 2014;21(8):768-83. doi: 10.5551/jat.23697. Epub 2014 Mar 12. J Atheroscler Thromb. 2014. PMID: 24621786 - NEMO modulates radiation-induced endothelial senescence of human umbilical veins through NF-κB signal pathway.
Dong X, Tong F, Qian C, Zhang R, Dong J, Wu G, Hu Y. Dong X, et al. Radiat Res. 2015 Jan;183(1):82-93. doi: 10.1667/RR13682.1. Epub 2014 Dec 23. Radiat Res. 2015. PMID: 25536232 - Downregulation of cathepsin C alleviates endothelial cell dysfunction by suppressing p38 MAPK/NF-κB pathway in preeclampsia.
Lu F, Gong H, Lei H, Li J. Lu F, et al. Bioengineered. 2022 Feb;13(2):3019-3028. doi: 10.1080/21655979.2021.2023994. Bioengineered. 2022. PMID: 35037834 Free PMC article.
Cited by
- PEDF promotes the repair of bone marrow endothelial cell injury and accelerates hematopoietic reconstruction after bone marrow transplantation.
Ju W, Lu W, Ding L, Bao Y, Hong F, Chen Y, Gao H, Xu X, Wang G, Wang W, Zhang X, Fu C, Qi K, Li Z, Xu K, Qiao J, Zeng L. Ju W, et al. J Biomed Sci. 2020 Sep 1;27(1):91. doi: 10.1186/s12929-020-00685-4. J Biomed Sci. 2020. PMID: 32873283 Free PMC article. - Zonula Occludens Proteins Signaling in Inflammation and Tumorigenesis.
Yu S, He J, Xie K. Yu S, et al. Int J Biol Sci. 2023 Jul 24;19(12):3804-3815. doi: 10.7150/ijbs.85765. eCollection 2023. Int J Biol Sci. 2023. PMID: 37564207 Free PMC article. Review. - Heyingwuzi formulation alleviates diabetic retinopathy by promoting mitophagy via the HIF-1α/BNIP3/NIX axis.
Wu JJ, Zhang SY, Mu L, Dong ZG, Zhang YJ. Wu JJ, et al. World J Diabetes. 2024 Jun 15;15(6):1317-1339. doi: 10.4239/wjd.v15.i6.1317. World J Diabetes. 2024. PMID: 38983802 Free PMC article. - Lyophilized Progenitor Tenocyte Extracts: Sterilizable Cytotherapeutic Derivatives with Antioxidant Properties and Hyaluronan Hydrogel Functionalization Effects.
Laurent A, Porcello A, Jeannerat A, Peneveyre C, Coeur A, Abdel-Sayed P, Scaletta C, Michetti M, de Buys Roessingh A, Jordan O, Allémann E, Raffoul W, Hirt-Burri N, Applegate LA. Laurent A, et al. Antioxidants (Basel). 2023 Jan 10;12(1):163. doi: 10.3390/antiox12010163. Antioxidants (Basel). 2023. PMID: 36671025 Free PMC article. - DNA damage response in vascular endothelial senescence: Implication for radiation-induced cardiovascular diseases.
Nagane M, Yasui H, Kuppusamy P, Yamashita T, Inanami O. Nagane M, et al. J Radiat Res. 2021 Jul 10;62(4):564-573. doi: 10.1093/jrr/rrab032. J Radiat Res. 2021. PMID: 33912932 Free PMC article. Review.
References
- Schollnberger H., Eidemuller M., Cullings H. M., Simonetto C., Neff F., Kaiser J. C. Dose-responses for mortality from cerebrovascular and heart diseases in atomic bomb survivors: 1950–2003. Radiation and Environmental Biophysics. 2018;57(1):17–29. doi: 10.1007/s00411-017-0722-5. - DOI - PMC - PubMed
- Gabrys D., Greco O., Patel G., Prise K. M., Tozer G. M., Kanthou C. Radiation effects on the cytoskeleton of endothelial cells and endothelial monolayer permeability. International Journal of Radiation Oncology, Biology, Physics. 2007;69(5):1553–1562. doi: 10.1016/j.ijrobp.2007.08.039. - DOI - PubMed
- Ungvari Z., Podlutsky A., Sosnowska D., et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. The Journals of Gerontology: Series A. 2013;68(12):1443–1457. doi: 10.1093/gerona/glt057. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous