miR-26b enhances the sensitivity of hepatocellular carcinoma to Doxorubicin via USP9X-dependent degradation of p53 and regulation of autophagy - PubMed (original) (raw)
. 2021 Feb 8;17(3):781-795.
doi: 10.7150/ijbs.52517. eCollection 2021.
Affiliations
- PMID: 33767588
- PMCID: PMC7975695
- DOI: 10.7150/ijbs.52517
miR-26b enhances the sensitivity of hepatocellular carcinoma to Doxorubicin via USP9X-dependent degradation of p53 and regulation of autophagy
Enjiang Chen et al. Int J Biol Sci. 2021.
Abstract
Multi-drug resistance is a major challenge to hepatocellular carcinoma (HCC) treatment, and the over-expression or deletion of microRNA (miRNA) expression is closely related to the drug-resistant properties of various cell lines. However, the underlying molecular mechanisms remain unclear. CCK-8, EdU, flow cytometry, and transmission electron microscopy were performed to determine cell viability, proliferation, apoptosis, autophagic flow, and nanoparticle characterization, respectively. In this study, the results showed that the expression of miR-26b was downregulated following doxorubicin treatment in human HCC tissues. An miR-26b mimic enhanced HCC cell doxorubicin sensitivity, except in the absence of p53 in Hep3B cells. Delivery of the proteasome inhibitor, MG132, reversed the inhibitory effect of miR-26b on the level of p53 following doxorubicin treatment. Tenovin-1 (an MDM2 inhibitor) protected p53 from ubiquitination-mediated degradation only in HepG2 cells with wild type p53. Tenovin-1 pretreatment enhanced HCC cell resistance to doxorubicin when transfected with an miR-26b mimic. Moreover, the miR-26b mimic inhibited doxorubicin-induced autophagy and the autophagy inducer, rapamycin, eliminated the differences in the drug sensitivity effect of miR-26b. In vivo, treatment with sp94dr/miR-26b mimic nanoparticles plus doxorubicin inhibited tumor growth. Our current data indicate that miR-26b enhances HCC cell sensitivity to doxorubicin through diminishing USP9X-mediated p53 de-ubiquitination caused by DNA damaging drugs and autophagy regulation. This miRNA-mediated pathway that modulates HCC will help develop novel therapeutic strategies.
Keywords: Doxorubicin; USP9X; hepatocellular carcinoma; microRNA-26b; p53..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Figure 1
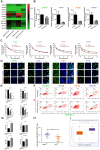
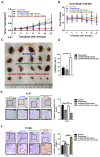
miR-26b enhances HCC cell sensitivity to doxorubicin. A. QRT-PCR analysis of the changes in miRNA after treatment with doxorubicin in SNU449 and SNU387 cells. B. The level of miR-26b was determined following treatment with or without Doxorubicin by qRT-PCR in HCC cells. **P < 0.01. C. A CCK-8 assay analysis showed that treatment with an miR-26b mimic can enhance the sensitivity of HCC cells to doxorubicin, with the exception of Hep3B cells. D and E. An EdU incorporation assay of cellular proliferation in different treatment groups. **P < 0.01. F and G. The apoptosis ratio was determined by flow cytometry. *P < 0.05; **P < 0.01. H. QRT-PCR used to determine miR-26b expression in adjacent cancer and adjacent tissues. I. We used StarBase v 3.0 project to analyze the level of miR-26b in normal and cancer tissues.
Figure 2
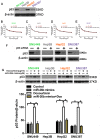
p53 was related to the sensitivity of HCC cells to doxorubicin. A. P53 protein expression was detected by Western blot. B - E. The cell viability was examined in doxorubicin, or miR-26b mimic plus doxorubicin treated cells following transfection with p53 siRNA.
Figure 3
miR-26b enhances HCC cell sensitivity to doxorubicin via USP9X-dependent p53 degradation. A. Western blot indicating the expression of p53 after treated with doxorubicin, doxorubicin plus miR-26 mimic, or MG132. **P < 0.01, ***P < 0.001. B. Tenovin-1 combined with an miR-26b mimic could enhance doxorubicin sensitivity in HepG2 cells, except other HCC cells. C. The predicted miR-26b binding site in the USP9X 3′ UTR and dual fluorescence reporter gene experiments verify that miR-26b binds to the promoter region of USP9X. *P < 0.05, **P < 0.01, ***P < 0.001. D. The expression of USP9X was up-regulated following treatment with the miR-26b mimic. *P < 0.05, **P < 0.01. E. P53 protein expression was determined by Western blot. F. The expression of UXP9X was determined by Western Blot.
Figure 4
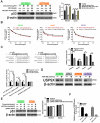
miR-26b enhances doxorubicin sensitivity via autophagy. A. The expression of proteins in HCC cells in different treatment groups detected by Western blot. _*_P < 0.05, _**_P < 0.01.B-D. Confocal microscopy analysis of LC3 double fluorescent cells. *P < 0.05; **P < 0.01; ***P < 0.001. E-F. Electron microscopy detected autophagic flow in SNU449 and HepG2 cells. **P < 0.01 G. CCK-8 detected the sensitivity to doxorubicin in HCC cells after treatment with different conditions (transfected with miR-26b mimics, miR-26b mimic interference, and treated with rapamycin). *P < 0.05 vs Negative Control.
Figure 5
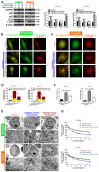
Treatment with an miR-26b mimic inhibits tumor growth in vivo. A. Growth curves of xenograft tumors treated with the control, doxorubicin, sp-94dr/miR-26b mimic, or doxorubicin plus with sp-94dr/miR-26b mimic (n = 6 per group). B. The mice were weighed on days 0-15 following treatment with either the control, doxorubicin, sp-94dr/miR-26b mimic, or doxorubicin plus with sp-94dr/miR-26b mimic. C. The morphology of the subcutaneous xenograft tumors in each of the different treatment groups. D. The tumor inhibition rate was determined following treatment with doxorubicin, sp-94dr/miR-26b mimic, or doxorubicin plus with sp-94dr/miR-26b mimic. *P < 0.05. E. Ki-67 staining was used to analyze the rate of Ki-67-positive staining in each of the treatment groups (40× magnification). *P < 0.05; **P < 0.01 F. The presence of apoptotic cells was detected in each of the treatment groups by a TUNEL assay. *P < 0.05; ***P < 0.001**.**
Figure 6
Treatment with Sp-94dr/miR-26b mimic combined with doxorubicin down-regulates p53 and USP9X expression in vivo. A and B. Immunohistochemistry-positive (IHC-P) cells were examined for the expression of USP9X and p53. *P < 0.05; **P < 0.01; ***P < 0.001. C. QRT-PCR analysis the expression of miR-26b, USP9X, and p53 in control, doxorubicin, sp-94dr/miR-26b mimic, and doxorubicin plus with sp-94dr/miR-26b mimic-treated cells. *P < 0.05; **P < 0.01; ***P < 0.001. D. USP9X, p53, and p62 protein expression were evaluated using a Western blot. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 7
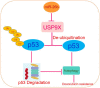
Schematic diagram of the regulatory mechanism of the miR-26b/USP9X/p53 axis in regulating HCC sensitivity to doxorubicin.
Similar articles
- WP1130 increases doxorubicin sensitivity in hepatocellular carcinoma cells through usp9x-dependent p53 degradation.
Liu H, Chen W, Liang C, Chen BW, Zhi X, Zhang S, Zheng X, Bai X, Liang T. Liu H, et al. Cancer Lett. 2015 Jun 1;361(2):218-25. doi: 10.1016/j.canlet.2015.03.001. Epub 2015 Mar 5. Cancer Lett. 2015. PMID: 25749422 - MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy.
Jin F, Wang Y, Li M, Zhu Y, Liang H, Wang C, Wang F, Zhang CY, Zen K, Li L. Jin F, et al. Cell Death Dis. 2017 Jan 12;8(1):e2540. doi: 10.1038/cddis.2016.461. Cell Death Dis. 2017. PMID: 28079894 Free PMC article. - MicroRNA-26b inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting USP9X.
Shen G, Lin Y, Yang X, Zhang J, Xu Z, Jia H. Shen G, et al. BMC Cancer. 2014 Jun 2;14:393. doi: 10.1186/1471-2407-14-393. BMC Cancer. 2014. PMID: 24890815 Free PMC article. - MiR-34a overexpression enhances the inhibitory effect of doxorubicin on HepG2 cells.
Zheng SZ, Sun P, Wang JP, Liu Y, Gong W, Liu J. Zheng SZ, et al. World J Gastroenterol. 2019 Jun 14;25(22):2752-2762. doi: 10.3748/wjg.v25.i22.2752. World J Gastroenterol. 2019. PMID: 31235998 Free PMC article. - Advancements in Utilizing Natural Compounds for Modulating Autophagy in Liver Cancer: Molecular Mechanisms and Therapeutic Targets.
Rahman MA, Rakib-Uz-Zaman SM, Chakraborti S, Bhajan SK, Gupta RD, Jalouli M, Parvez MAK, Shaikh MH, Hoque Apu E, Harrath AH, Moon S, Kim B. Rahman MA, et al. Cells. 2024 Jul 12;13(14):1186. doi: 10.3390/cells13141186. Cells. 2024. PMID: 39056768 Free PMC article. Review.
Cited by
- Targeting and regulation of autophagy in hepatocellular carcinoma: revisiting the molecular interactions and mechanisms for new therapy approaches.
Hashemi M, Nadafzadeh N, Imani MH, Rajabi R, Ziaolhagh S, Bayanzadeh SD, Norouzi R, Rafiei R, Koohpar ZK, Raei B, Zandieh MA, Salimimoghadam S, Entezari M, Taheriazam A, Alexiou A, Papadakis M, Tan SC. Hashemi M, et al. Cell Commun Signal. 2023 Feb 9;21(1):32. doi: 10.1186/s12964-023-01053-z. Cell Commun Signal. 2023. PMID: 36759819 Free PMC article. Review. - Oxygen microcapsules improve immune checkpoint blockade by ameliorating hypoxia condition in pancreatic ductal adenocarcinoma.
Wu J, Wang X, Chen L, Wang J, Zhang J, Tang J, Ji Y, Song J, Wang L, Zhao Y, Zhang H, Li T, Sheng J, Chen D, Zhang Q, Liang T. Wu J, et al. Bioact Mater. 2022 Jun 2;20:259-270. doi: 10.1016/j.bioactmat.2022.05.022. eCollection 2023 Feb. Bioact Mater. 2022. PMID: 35702611 Free PMC article. - Systematic assessment of microRNAs associated with lung cancer and physical exercise.
Liu Y, He L, Wang W. Liu Y, et al. Front Oncol. 2022 Aug 30;12:917667. doi: 10.3389/fonc.2022.917667. eCollection 2022. Front Oncol. 2022. PMID: 36110941 Free PMC article. - Loss of miR-26b-5p promotes gastric cancer progression via miR-26b-5p-PDE4B/CDK8-STAT3 feedback loop.
Xu T, Xie M, Jing X, Jiang H, Wu X, Wang X, Shu Y. Xu T, et al. J Transl Med. 2023 Feb 3;21(1):77. doi: 10.1186/s12967-023-03933-x. J Transl Med. 2023. PMID: 36737782 Free PMC article. - Little things with significant impact: miRNAs in hepatocellular carcinoma.
Li J, Bao H, Huang Z, Liang Z, Wang M, Lin N, Ni C, Xu Y. Li J, et al. Front Oncol. 2023 May 19;13:1191070. doi: 10.3389/fonc.2023.1191070. eCollection 2023. Front Oncol. 2023. PMID: 37274242 Free PMC article. Review.
References
- Gao J, Inagaki Y, Song P, Qu X, Kokudo N, Tang W. Targeting c-Met as a promising strategy for the treatment of hepatocellular carcinoma. Pharmacol Res. 2012;65:23–30. - PubMed
- Ma J, Zeng S, Zhang Y, Deng G, Qu Y, Guo C. et al. BMP4 promotes oxaliplatin resistance by an induction of epithelial-mesenchymal transition via MEK1/ERK/ELK1 signaling in hepatocellular carcinoma. Cancer Lett. 2017;411:117–29. - PubMed
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous