American Society of Hematology 2023 guidelines for management of venous thromboembolism: thrombophilia testing - PubMed (original) (raw)
. 2023 Nov 28;7(22):7101-7138.
doi: 10.1182/bloodadvances.2023010177.
Robby Nieuwlaat 2 3, Lisa Baumann Kreuziger 4, Michiel Coppens 5 6, Damon Houghton 7 8, Andra H James 9, Eddy Lang 10, Stephan Moll 11, Tarra Myers 12, Meha Bhatt 2, Chatree Chai-Adisaksopha 13, Luis E Colunga-Lozano 14, Samer G Karam 2 3, Yuan Zhang 1 2, Wojtek Wiercioch 2 3, Holger J Schünemann 2 3 15, Alfonso Iorio 3
Affiliations
- PMID: 37195076
- PMCID: PMC10709681
- DOI: 10.1182/bloodadvances.2023010177
American Society of Hematology 2023 guidelines for management of venous thromboembolism: thrombophilia testing
Saskia Middeldorp et al. Blood Adv. 2023.
Abstract
Hereditary and acquired thrombophilia are risk factors for venous thromboembolism (VTE). Whether testing helps guide management decisions is controversial. These evidence-based guidelines from the American Society of Hematology (ASH) intend to support decision making about thrombophilia testing. ASH formed a multidisciplinary guideline panel covering clinical and methodological expertise and minimizing bias from conflicts of interest. The McMaster University GRADE Centre provided logistical support, performed systematic reviews, and created evidence profiles and evidence-to-decision tables. The Grading of Recommendations Assessment, Development, and Evaluation approach (GRADE) was used. Recommendations were subject to public comment. The panel agreed on 23 recommendations regarding thrombophilia testing and associated management. Nearly all recommendations are based on very low certainty in the evidence due to modeling assumptions. The panel issued a strong recommendation against testing the general population before starting combined oral contraceptives (COCs) and conditional recommendations for thrombophilia testing in the following scenarios: (a) patients with VTE associated with nonsurgical major transient or hormonal risk factors; (b) patients with cerebral or splanchnic venous thrombosis, in settings where anticoagulation would otherwise be discontinued; (c) individuals with a family history of antithrombin, protein C, or protein S deficiency when considering thromboprophylaxis for minor provoking risk factors and for guidance to avoid COCs/hormone replacement therapy; (d) pregnant women with a family history of high-risk thrombophilia types; and (e) patients with cancer at low or intermediate risk of thrombosis and with a family history of VTE. For all other questions, the panel provided conditional recommendations against testing for thrombophilia.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: All authors were members of either the guideline panel or of the systematic review team. Consequently, they completed a disclosure-of-interest form, which was reviewed by ASH and is available as Supplements 2 and 3.
Figures
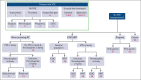
Figure 1.
Overview of guideline questions. Minor provoking risk factors: circumstances that generally do not require prophylaxis, such as immobility or minor injury, illness, or infection. RF, risk factor.
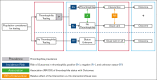
Figure 2.
Modeling approach for determining the effect of thrombophilia testing. Population considered for testing: Figure 1 with the guideline flowchart for the different populations for which a recommendation regarding thrombophilia testing was provided. Thrombophilia: any type of thrombophilia or a specific type, depending on whether the recommendation question addresses panel testing or testing for a known specific type in the family. Intervention: course of action other than “usual care.” Depending on the specific question, this means prescribing thromboprophylaxis, withholding thromboprophylaxis, extending thromboprophylaxis, stopping thromboprophylaxis, withholding COCs, or withholding HRT. Usual care: for populations where “usual care” was ambiguous, 2 scenarios were modeled, and separate recommendations were provided (see recommendations 7-10).
Comment in
- Commentary on the 2023 ASH guidelines for thrombophilia testing in venous thromboembolism.
Juneja M, Szer J. Juneja M, et al. Blood Adv. 2023 Nov 14;7(21):6428-6429. doi: 10.1182/bloodadvances.2023011393. Blood Adv. 2023. PMID: 37581979 Free PMC article. No abstract available. - Thrombophilia management calculator.
Hozo I, Djulbegovic B. Hozo I, et al. Blood Adv. 2024 Aug 13;8(15):3914-3916. doi: 10.1182/bloodadvances.2024013463. Blood Adv. 2024. PMID: 38759098 Free PMC article. No abstract available.
Similar articles
- American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy.
Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, Vazquez SR, Greer IA, Riva JJ, Bhatt M, Schwab N, Barrett D, LaHaye A, Rochwerg B. Bates SM, et al. Blood Adv. 2018 Nov 27;2(22):3317-3359. doi: 10.1182/bloodadvances.2018024802. Blood Adv. 2018. PMID: 30482767 Free PMC article. - American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism.
Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, Chan AKC, Hanson S, Male C, Meerpohl J, Newall F, O'Brien SH, Raffini L, van Ommen H, Wiernikowski J, Williams S, Bhatt M, Riva JJ, Roldan Y, Schwab N, Mustafa RA, Vesely SK. Monagle P, et al. Blood Adv. 2018 Nov 27;2(22):3292-3316. doi: 10.1182/bloodadvances.2018024786. Blood Adv. 2018. PMID: 30482766 Free PMC article. - Joint 2022 European Society of Thoracic Surgeons and The American Association for Thoracic Surgery guidelines for the prevention of cancer-associated venous thromboembolism in thoracic surgery.
Shargall Y, Wiercioch W, Brunelli A, Murthy S, Hofstetter W, Lin J, Li H, Linkins LA, Crowther M, Davis R, Rocco G, Morgano GP, Schünemann F, Muti-Schünemann G, Douketis J, Schünemann HJ, Litle VR. Shargall Y, et al. J Thorac Cardiovasc Surg. 2023 Mar;165(3):794-824.e6. doi: 10.1016/j.jtcvs.2022.05.041. Epub 2022 Dec 15. J Thorac Cardiovasc Surg. 2023. PMID: 36895083 - Thrombophilia Testing in Venous Thromboembolism.
Vrotniakaite-Bajerciene K, Angelillo-Scherrer A, Castellucci LA. Vrotniakaite-Bajerciene K, et al. Med Clin North Am. 2025 Jul;109(4):907-921. doi: 10.1016/j.mcna.2025.01.008. Epub 2025 Apr 12. Med Clin North Am. 2025. PMID: 40500088 Review.
Cited by
- Predicting inferior vena cava filter complications using machine learning.
Li B, Eisenberg N, Beaton D, Lee DS, Al-Omran L, Wijeysundera DN, Hussain MA, Rotstein OD, de Mestral C, Mamdani M, Roche-Nagle G, Al-Omran M. Li B, et al. J Vasc Surg Venous Lymphat Disord. 2024 Nov;12(6):101943. doi: 10.1016/j.jvsv.2024.101943. Epub 2024 Jul 29. J Vasc Surg Venous Lymphat Disord. 2024. PMID: 39084408 Free PMC article. - Development and validation of a genetic probability for venous thromboembolism.
Shi Z, Mulford AJ, Tran H, Filipczak M, Gao S, Xie S, Lobel J, Wei J, Rifkin AS, Ashworth A, Zheng SL, Wiske C, Duggan D, Helfand BT, Qamar A, Sanders AR, Tafur AJ, Xu J. Shi Z, et al. Res Pract Thromb Haemost. 2025 Apr 27;9(4):102876. doi: 10.1016/j.rpth.2025.102876. eCollection 2025 May. Res Pract Thromb Haemost. 2025. PMID: 40496845 Free PMC article. - Deciphering the Complex Relationships Between the Hemostasis System and Infective Endocarditis.
Wahab MA, Khan AU, Mercadante S, Cafarella I, Bertolino L, Durante-Mangoni E. Wahab MA, et al. J Clin Med. 2025 Jun 4;14(11):3965. doi: 10.3390/jcm14113965. J Clin Med. 2025. PMID: 40507729 Free PMC article. Review. - Thrombotic, Cardiovascular, and Microvascular Complications of Myeloproliferative Neoplasms and Clonal Hematopoiesis (CHIP): A Narrative Review.
Schafer AI, Mann DL. Schafer AI, et al. J Clin Med. 2024 Oct 12;13(20):6084. doi: 10.3390/jcm13206084. J Clin Med. 2024. PMID: 39458034 Free PMC article. Review. - Hormone-related thrombosis: duration of anticoagulation, risk of recurrence, and the role of hypercoagulability testing.
Scheres LJJ, Middeldorp S. Scheres LJJ, et al. Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):664-671. doi: 10.1182/hematology.2024000593. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39644057 Free PMC article. Review.
References
- Institute of Medicine . National Academies Press; 2011. Clinical Practice Guidelines We Can Trust. - PubMed
- Qaseem A, Forland F, Macbeth F, et al. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525–531. - PubMed
- Schunemann HJ, Al-Ansary LA, Forland F, et al. Guidelines International Network: principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med. 2015;163(7):548–553. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous