Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells - PubMed (original) (raw)
Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells
T S Migone et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Interleukin 2 (IL-2) rapidly induces tyrosine phosphorylation of intracellular substrates, including the IL-2 receptor beta chain (IL-2Rbeta), Janus kinase 1 (Jak1), Jak3, signal transducer/activator of transcription proteins, and Shc, but the mechanism underlying dephosphorylation of these proteins is not known. The src homology 2 (SH2) containing tyrosine phosphatase 1 (SHP-1) is recruited by several hematopoietic surface receptors indicating that this phosphatase plays an important role as a regulator of signaling. We have found that IL-2 induces association of SHP-1 with the IL-2 receptor complex, and that once SHP-1 is recruited to the activated receptor it is able to decrease tyrosine phosphorylation of IL-2Rbeta and the associated tyrosine kinases Jak1 and Jak3. This dephosphorylation is specific as expression of a catalytically inactive form of SHP-1, or expression of the related phosphatase SHP-2 did not result in dephosphorylation of the IL-2 receptor components. Furthermore, we have found that SHP-1 expression is greatly decreased or undetectable in a number of IL-2 independent HTLV-I transformed T cell lines that exhibit constitutive Jak/signal transducer/activator of transcription activation. In HTLV-I infected T cells, down-regulation of SHP-1 expression was also found to correlate with the acquisition of IL-2 independence. These observations suggest that SHP-1 normally functions to antagonize the IL-2 signal transduction pathway and that HTLV-I infection and oncogenic transformation can lead to loss of SHP-1 expression resulting in constitutive activation of IL-2 regulated T cell responses.
Figures
Figure 1
Time course of tyrosine phosphorylation of IL-2 receptor components after IL-2 stimulation. Molt4β cells were stimulated for 10′ with 2nM IL-2 at 37°C. Cells were washed in PBS and resuspended in 10% FBS/RPMI 1640 medium and left in culture for the indicated time. In control lanes (marked c), cells were not exposed to IL-2. Cells were then washed, lysed and immunoprecipitations were performed with the indicated antibodies: (A) anti-Jak 1; (B) anti-Jak3; (C) anti-IL-2Rβ and were followed by Western blot analysis with mAb 4G10. Immunoblots were stripped and reprobed to control for equal protein loading.
Figure 2
IL-2 induces association of SHP-1 with IL-2Rβ. (A) Molt4β cells were either stimulated with 2nM IL-2 for the indicated time or (B) stimulated for 10′ with 2nM IL-2, washed and resuspended in 10% FBS/RPMI 1640 medium in the absence of IL-2 for the indicated time. Cells were then washed, lysed and immunoprecipitations were performed with mAb 561 to hIL-2Rβ, followed by immunoblotting with anti-SHP-1 (Upstate Biotechnology). Immunoblots were then stripped and reprobed with an antiserum to human IL-2Rβ (Santa Cruz Biotechnology). (C) Freshly isolated peripheral blood mononuclear cells and Kit-225 cells were rested for 4 hr and then stimulated for 10′ with 2nM IL-2 at 37°C. Immunoprecipitations and Western blot analyses were performed as above.
Figure 3
SHP- 1 expression down-regulates tyrosine phosphorylation of IL-2 receptor components. (A) 293T+ cells were transfected with the indicated combination of plasmids; 48 hr posttransfection cells were washed, lysed and proteins were immunoprecipitated with mAb 561 to IL-2Rβ or (B) with antibodies to Jak1 and Jak3, followed by Western blot analysis with antiphosphotyrosine mAb. Also shown are controls for equal expression of the transfected plasmids. (C) A catalytically inactive form of SHP-1 does not affect tyrosine phosphorylation of the IL-2 receptor complex. 293 T+ cells were transfected with cDNAs for IL-2Rβ, γc, Jak1, Jak3, and either empty vector or the indicated forms of SHP-1 or SHP-2. Immunoprecipitations and immunoblotting were performed as described in A. Also shown are controls for equal expression of the transfected plasmids.
Figure 4
SHP-1 expression down regulates IL-2 induced phosphorylation of Jak1 and Jak3 in αβγ-3T3 cells, but does not affect their ability to associate with IL-2Rβ. (A) αβγ-3T3 cells were infected with virus encoding wild-type Jak3 (lanes 1 and 2), kinase inactive Jak3 (lanes 3 and 4), and wild-type Jak3 and SHP-1 (lanes 5 and 6). Cells were stimulated for 30 min with 2nM IL-2 at 37°C. Immunoprecipitations and Western blot analysis were performed as above. (B) The membrane was stripped and reprobed with anti Jak1 and anti-Jak3 antibody to analyze the effect of SHP-1 on IL-2Rβ-Jak1 interactions. Additionally, the same membrane was reprobed with anti-IL-2Rβ antibody as a control for equal protein loading.
Figure 5
IL-2 receptor phosphorylation in HTLV-I-transformed T cells. The HTLV-I-transformed T cell line MT-2 was stimulated as described in Fig. 1. Immunoprecipitations and Western blot analysis were performed as described in Fig. 1.
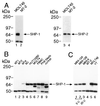
Figure 6
SHP-1 expression is not detected in some HTLV-I-transformed T cell lines. (A) Lysates (40 μg) from MOLT4β or MT-2 cells probed with antisera to either SHP-1 or SHP-2. (B) Lysates (40 μg) from the HTLV-I-transformed T cell lines MT1, MT2, MT4, MJ, Hut102B, or from MOLT4β, freshly isolated peripheral blood mononuclear cells, phytohemagglutinin-activated peripheral blood lymphocytes, and Jurkat, were immunoblotted with anti-SHP-1. (C) 40 μg of lysates from the HTLV-I-infected T cells MB3–12A, MB3–12B (grown in medium containing 3 units IL-2/ml), MB3–6 (grown in medium containing 20 units IL-2/ml) were compared with lysates from MT-2, MOLT4β, and YT cells by immunoblotting with anti-SHP-1.
Similar articles
- Differences in phosphorylation of the IL-2R associated JAK/STAT proteins between HTLV-I(+), IL-2-independent and IL-2-dependent cell lines and uncultured leukemic cells from patients with adult T-cell lymphoma/leukemia.
Zhang Q, Lee B, Korecka M, Li G, Weyland C, Eck S, Gessain A, Arima N, Lessin SR, Shaw LM, Luger S, Kamoun M, Wasik MA. Zhang Q, et al. Leuk Res. 1999 Apr;23(4):373-84. doi: 10.1016/s0145-2126(98)00173-8. Leuk Res. 1999. PMID: 10229324 - Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling.
Zhang J, Somani AK, Siminovitch KA. Zhang J, et al. Semin Immunol. 2000 Aug;12(4):361-78. doi: 10.1006/smim.2000.0223. Semin Immunol. 2000. PMID: 10995583 Review. - Cytoplasmic protein tyrosine phosphatases SHP-1 and SHP-2: regulators of B cell signal transduction.
Tamir I, Dal Porto JM, Cambier JC. Tamir I, et al. Curr Opin Immunol. 2000 Jun;12(3):307-15. doi: 10.1016/s0952-7915(00)00092-3. Curr Opin Immunol. 2000. PMID: 10781410 Review.
Cited by
- Consideration of SHP-1 as a Molecular Target for Tumor Therapy.
Lim S, Lee KW, Kim JY, Kim KD. Lim S, et al. Int J Mol Sci. 2023 Dec 26;25(1):331. doi: 10.3390/ijms25010331. Int J Mol Sci. 2023. PMID: 38203502 Free PMC article. Review. - HTLV-1 Tax Tug-of-War: Cellular Senescence and Death or Cellular Transformation.
Bellon M, Nicot C. Bellon M, et al. Pathogens. 2024 Jan 19;13(1):87. doi: 10.3390/pathogens13010087. Pathogens. 2024. PMID: 38276160 Free PMC article. Review. - Epigenetic mechanisms of protein tyrosine phosphatase 6 suppression in diffuse large B-cell lymphoma: implications for epigenetic therapy.
Witzig TE, Hu G, Offer SM, Wellik LE, Han JJ, Stenson MJ, Dogan A, Diasio RB, Gupta M. Witzig TE, et al. Leukemia. 2014 Jan;28(1):147-54. doi: 10.1038/leu.2013.251. Epub 2013 Aug 27. Leukemia. 2014. PMID: 23979523 Free PMC article. - Mechanisms of SHP-1 P2 promoter regulation in hematopoietic cells and its silencing in HTLV-1-transformed T cells.
Nakase K, Cheng J, Zhu Q, Marasco WA. Nakase K, et al. J Leukoc Biol. 2009 Jan;85(1):165-74. doi: 10.1189/jlb.0608383. Epub 2008 Nov 12. J Leukoc Biol. 2009. PMID: 18948549 Free PMC article. - IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells.
Gasteiger G, Hemmers S, Firth MA, Le Floc'h A, Huse M, Sun JC, Rudensky AY. Gasteiger G, et al. J Exp Med. 2013 Jun 3;210(6):1167-78. doi: 10.1084/jem.20122462. Epub 2013 May 6. J Exp Med. 2013. PMID: 23650441 Free PMC article.
References
- Leonard W J. Annu Rev Med. 1996;47:229–236. - PubMed
- Taniguchi T. Science. 1995;268:251–255. - PubMed
- Johnston J A, Kawamura M, Kirken R A, Chen Y Q, Blake T B, Shibuya K, Ortaldo J R, McVicar D W, O’Shea J J. Nature (London) 1994;370:151–153. - PubMed
- Russell S M, Johnston J A, Noguchi M, Kawamura M, Bacon C M, Friedmann M, Berg M, McVicar D W, Witthun B A, Silvennoinen O A S, et al. Science. 1994;266:1042–1045. - PubMed
- Boussiotis V A, Barber D L, Nakarai T, Freeman G J, Gribben J G, Bernstein G M, D’Andrea A D, Ritz J, Nadler L M. Science. 1994;266:1039–1042. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous