Ammonia - NH3 - Thermodynamic Properties (original) (raw)
Thermodynamic properties of saturated and superheated ammonia R-717 like specific volume, enthalpy and entropy.
Specific volume (v), specific internal energy (u), enthalpy (h), and entropy (s) of saturated and superheated ammonia - NH3 - also known as refrigerant 717.
For full table with Specific Entropy and Superheated Properties - rotate the screen!
Ammonia - NH3 - Thermodynamic Properties
| Saturated Properties | Superheated Properties (t - ts) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
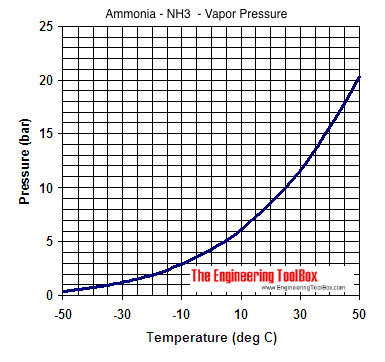
| Temperature - ts - (oC) | Pressure - p s - (bar) | Specific Volume - vi - (m3/kg) | Specific Enthalpy | Specific Entropy | Abs. Temperature (50 K) | Abs. Temperature (100 K) | ||||
| saturated liquid - hf - (kJ/kg) | saturated vapor - hg - (kJ/kg) | saturated liquid - sf - (kJ/kgK) | saturated vapor - sg - (kJ/kgK) | Specific Enthalpy - h - (kJ/kg) | Specific Entropy - s - (kJ/kgK) | Specific Enthalpy - h - (kJ/kg) | Specific Entropy - s - (kJ/kgK) | |||
| -50 | 0.4089 | 2.625 | -44.4 | 1373.3 | -0.194 | 6.159 | 1479.8 | 6.592 | 1585.9 | 6.948 |
| -45 | 0.5454 | 2.005 | -22.3 | 1381.6 | -0.096 | 6.057 | 1489.3 | 6.486 | 1596.1 | 6.839 |
| -40 | 0.7177 | 1.552 | 0 | 1390.0 | 0 | 5.962 | 1498.6 | 6.387 | 1606.3 | 6.736 |
| -35 | 0.9322 | 1.216 | 22.3 | 1397.9 | 0.095 | 5.872 | 1507.9 | 6.293 | 1616.3 | 6.639 |
| -30 | 1.196 | 0.9633 | 44.7 | 1405.6 | 0.188 | 5.785 | 1517.0 | 6.203 | 1626.3 | 6.547 |
| -28 | 1.317 | 0.8809 | 53.6 | 1408.5 | 0.224 | 5.751 | 1520.7 | 6.169 | 1630.3 | 6.512 |
| -26 | 1.447 | 0.8058 | 62.6 | 1411.4 | 0.261 | 5.718 | 1524.3 | 6.135 | 1634.2 | 6.477 |
| -24 | 1.588 | 0.7389 | 71.7 | 1414.3 | 0.297 | 5.686 | 1527.9 | 6.103 | 1638.2 | 6.444 |
| -22 | 1.740 | 0.6783 | 80.8 | 1417.3 | 0.333 | 5.655 | 1531.4 | 6.071 | 1642.2 | 6.411 |
| -20 | 1.902 | 0.6237 | 89.8 | 1420.0 | 0.368 | 5.623 | 1534.8 | 6.039 | 1646.0 | 6.379 |
| -18 | 2.077 | 0.5743 | 98.8 | 1422.7 | 0.404 | 5.593 | 1538.2 | 6.008 | 1650.0 | 6.347 |
| -16 | 2.265 | 0.5296 | 107.9 | 1425.3 | 0.440 | 5.563 | 1541.7 | 5.978 | 1653.8 | 6.316 |
| -14 | 2.465 | 0.4890 | 117.0 | 1427.9 | 0.475 | 5.533 | 1545.1 | 5.948 | 1657.7 | 6.286 |
| -12 | 2.680 | 0.4521 | 126.2 | 1430.5 | 0.510 | 5.504 | 1548.5 | 5.919 | 1661.5 | 6.256 |
| -10 | 2.908 | 0.4185 | 135.4 | 1433.0 | 0.544 | 5.475 | 1551.7 | 5.891 | 1665.3 | 6.227 |
| -8 | 3.153 | 0.3879 | 144.5 | 1435.3 | 0.579 | 5.447 | 1554.9 | 5.863 | 1669.0 | 6.199 |
| -6 | 3.413 | 0.3599 | 153.6 | 1437.6 | 0.613 | 5.419 | 1558.2 | 5.836 | 1672.8 | 6.171 |
| -4 | 3.691 | 0.3344 | 162.8 | 1439.9 | 0.647 | 5.392 | 1561.4 | 5.808 | 1676.4 | 6.143 |
| -2 | 3.983 | 0.3110 | 172.0 | 1442.2 | 0.681 | 5.365 | 1564.6 | 5.782 | 1680.1 | 6.116 |
| 0 | 4.295 | 0.2895 | 181.2 | 1444.4 | 0.715 | 5.340 | 1567.8 | 5.756 | 1683.9 | 6.090 |
| 2 | 4.625 | 0.2699 | 190.4 | 1446.5 | 0.749 | 5.314 | 1570.9 | 5.731 | 1687.5 | 6.065 |
| 4 | 4.975 | 0.2517 | 199.7 | 1448.5 | 0.782 | 5.288 | 1574.0 | 5.706 | 1691.2 | 6.040 |
| 6 | 5.346 | 0.2351 | 209.1 | 1450.6 | 0.816 | 5.263 | 1577.0 | 5.682 | 1694.9 | 6.015 |
| 8 | 5.736 | 0.2198 | 218.5 | 1452.5 | 0.849 | 5.238 | 1580.1 | 5.658 | 1698.4 | 5.991 |
| 10 | 6.149 | 0.2056 | 227.8 | 1454.3 | 0.881 | 5.213 | 1583.1 | 5.634 | 1702.2 | 5.967 |
| 12 | 6.585 | 0.1926 | 237.2 | 1456.1 | 0.914 | 5.189 | 1586.0 | 5.611 | 1705.7 | 5.943 |
| 14 | 7.045 | 0.1805 | 246.6 | 1457.8 | 0.947 | 5.165 | 1588.9 | 5.588 | 1709.1 | 5.920 |
| 16 | 7.529 | 0.1693 | 256.0 | 1459.5 | 0.979 | 5.141 | 1591.7 | 5.565 | 1712.5 | 5.898 |
| 18 | 8.035 | 0.1590 | 265.5 | 1461.1 | 1.012 | 5.118 | 1594.4 | 5.543 | 1715.9 | 5.876 |
| 20 | 8.570 | 0.1494 | 275.1 | 1462.6 | 1.044 | 5.095 | 1597.2 | 5.521 | 1719.3 | 5.854 |
| 22 | 9.134 | 0.1405 | 284.6 | 1463.9 | 1.076 | 5.072 | 1600.0 | 5.499 | 1722.8 | 5.832 |
| 24 | 9.722 | 0.1322 | 294.1 | 1465.2 | 1.108 | 5.049 | 1602.7 | 5.478 | 1726.3 | 5.811 |
| 26 | 10.34 | 0.1245 | 303.7 | 1466.5 | 1.140 | 5.027 | 1605.3 | 5.458 | 1729.6 | 5.790 |
| 28 | 10.99 | 0.1173 | 313.4 | 1467.8 | 1.172 | 5.005 | 1608.0 | 5.437 | 1732.7 | 5.770 |
| 30 | 11.67 | 0.1106 | 323.1 | 1468.9 | 1.204 | 4.984 | 1610.5 | 5.417 | 1735.9 | 5.750 |
| 32 | 12.37 | 0.1044 | 332.8 | 1469.9 | 1.235 | 4.962 | 1613.0 | 5.397 | 1739.3 | 5.731 |
| 34 | 13.11 | 0.0986 | 342.5 | 1470.8 | 1.267 | 4.940 | 1615.4 | 5.378 | 1742.6 | 5.711 |
| 36 | 13.89 | 0.0931 | 352.3 | 1471.8 | 1.298 | 4.919 | 1617.8 | 5.358 | 1745.7 | 5.692 |
| 38 | 14.70 | 0.0880 | 362.1 | 1472.6 | 1.329 | 4.898 | 1620.1 | 5.340 | 1748.7 | 5.674 |
| 40 | 15.54 | 0.0833 | 371.9 | 1473.3 | 1.360 | 4.877 | 1622.4 | 5.321 | 1751.9 | 5.655 |
| 42 | 16.42 | 0.0788 | 381.8 | 1473.8 | 1.391 | 4.856 | 1624.6 | 5.302 | 1755.0 | 5.637 |
| 44 | 17.34 | 0.0746 | 391.8 | 1474.2 | 1.422 | 4.835 | 1626.8 | 5.284 | 1758.0 | 5.619 |
| 46 | 18.30 | 0.0706 | 401.8 | 1474.5 | 1.453 | 4.814 | 1629.0 | 5.266 | 1761.0 | 5.602 |
| 48 | 19.29 | 0.0670 | 411.9 | 1474.7 | 1.484 | 4.793 | 1631.1 | 5.248 | 1764.0 | 5.584 |
| 50 | 20.33 | 0.0635 | 421.9 | 1474.7 | 1.515 | 4.773 | 1633.1 | 5.230 | 1766.8 | 5.567 |
- Ammonia Molecular weight : 17.03 g/mol
- Ammonia Melting point : -78oC
- Ammonia Latent heat of fusion (1,013 bar, at triple point) : 331.37 kJ/kg
- Ammonia Liquid Density (1.013 bar at boiling point) : 682 kg/m3 (250 K : 669 kg/m3) (300 K : 600 kg/m3) (400 K : 346 kg/m3)
- Ammonia Liquid Specific Heat (cp) (250 K : 4.52 kJ/kg.K) (300 K : 4.75 kJ/kg.K) (400 K : 6.91 kJ/kg.K)
- Ammonia Liquid/gas equivalent (1.013 bar and 15oC (59oF)) : 947 vol/vol
- Ammonia Liquid Dynamic (Absolute) Viscosity (223K (-50oC): 3.061 10-4 Ns/m2) (273K (0oC): 2.388 10-4 Ns/m2) (323K (50oC): 1.862 10-4 Ns/m2)
- Ammonia Liquid Thermal Conductivity (250 K : 592 106 kW/m.K) (300 K : 477 106 kW/m.K) (400 K : 207 106 kW/m.K)
- Ammonia Boiling point (1.013 bar) : -33.5oC
- Ammonia Latent heat of vaporization (1.013 bar at boiling point) : 1371.2 kJ/kg
- Ammonia Vapor pressure (at 21oC or 70oF) : 8.88 bar
- Ammonia Critical point - Critical temperature : 132.4oC - Critical pressure : 112.8 bar
- Ammonia Gas Density (1.013 bar at boiling point) : 0.86 kg/m3
- Ammonia Gas Density (1.013 bar and 15oC (59oF)) : 0.73 kg/m3
- Ammonia Gas Compressibility Factor (Z) (the ratio of the actual volume of the gas to the volume determined according to the perfect gas law) (1.013 bar and 15oC (59oF)) : 0.9929
- Ammonia Gas Specific Gravity (air = 1) (1.013 bar and 21oC (70oF)) : 0.597
- Ammonia Gas Specific volume (1.013 bar and 21oC (70oF)) : 1.411 m3/kg
- Ammonia Gas Specific Heat at constant pressure (cp) (1.013 bar and 15oC (59oF)) : 0.037 kJ/(mol.K)
- Ammonia Gas Specific Heat at constant volume (c_v_) (1.013 bar and 15oC (59oF)) : 0.028 kJ/(mol.K)
- Ammonia Gas Ratio of Specific Heats (Gamma: cp/cv) (1.013 bar and 15oC (59oF)) : 1.309623
- Ammonia Gas Dynamic Viscosity (1.013 bar and 0oC (32oF)) : 0.000098 Poise
- Ammonia Gas Thermal conductivity (1.013 bar and 0oC (32oF)) : 22.19 mW/(m.K)
- Ammonia Gas Solubility in water (1.013 bar and 0oC (32oF)) : 862 vol/vol
- Ammonia Gas Auto ignition temperature : 630oC
Related Topics
Design of Air Conditioning systems - heating, cooling and dehumidification of indoor air for thermal comfort.
Related Documents
Online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging from -73 to 425°C (-100 to 800°F) at pressure ranging from 1 to 1000 bara (14.5 - 14500 psia) - SI and Imperial Units.
Ammonia and health symptoms - smell and threat to life.
Figures and table with changes in Prandtl number for ammonia with changes in temperature and pressure.
Figures and tables showing how the properties of liquid and gaseous ammonia changes along the boiling/condensation curve (temperature and pressure between triple point and critical point conditions). An ammonia phase diagram are included.
Online calculator, figures and tables showing specific heat, CP and CV, of gasous and liquid ammonia at temperatures ranging from -73 to 425°C (-100 to 800°F) at pressure ranging from 1 to 100 bara (14.5 - 1450 psia) - SI and Imperial Units.
Online calculator, figures and tables showing thermal conductivity of liquid and gaseous ammonia at temperatures ranging -70 to 425 °C (-100 to 800 °F) at atmospheric and higher pressure - Imperial and SI Units.
Chemical, Physical and Thermal Properties of Ammonia. Phase diagram included.
Figures and table with ammonia saturation pressure at boiling points, SI and Imperial units.
Online calculator with figures and tables showing density and specific weight of ammonia for temperatures ranging -50 to 425 °C (-50 to 800 °F) at atmospheric and higher pressure - Imperial and SI Units.
Autoignition temperatures and flash points (°C and °F) of different types of hydrocarbons with varying carbon numbers up to C12.
Density, specific heat, thermal conductivity, viscosity and Prandtls no. of liquid ammonia at saturation pressure.
Standardized enthalpies and entropies for some common substances.
Triple points for common substances.
About the Engineering ToolBox!
Privacy Policy
We don't collect information from our users. More about
We use a third-party to provide monetization technologies for our site. You can review their privacy and cookie policy here.
You can change your privacy settings by clicking the following button: .
Citation
This page can be cited as
- The Engineering ToolBox (2005). Ammonia - NH3 - Thermodynamic Properties. [online] Available at: https://www.engineeringtoolbox.com/ammonia-d\_971.html [Accessed Day Month Year].
Modify the access date according your visit.