Ammonia - Thermophysical Properties (original) (raw)
Ammonia is a colourless gas with a characteristic pungent smell and hazardous in its concentrated form.
Chemical, physical and thermal properties of Ammonia, NH3 :
Values at 25 oC (77 oF, 298 K) and 1 atm, if not other temperature and pressure given.
If values are given for liquid ammonia at ambient temperature, the ammonia is pressurized above 1 atm.
For full table with Imperial units - rotate the screen!
Ammonia - Thermophysical Properties
| Property | Value | Unit | Value | Unit | Value | Unit | Value | Unit |
|---|---|---|---|---|---|---|---|---|
| Acidity (pKa) | 9.24 | |||||||
| Acidity (pKa) at -33°C | 32.5 | |||||||
| Autoignition point | 903 | K | 630 | °C | 1166 | °F | ||
| Basicity (pKb) | 4.75 | |||||||
| Boiling point | 239.82 | K | -33.33 | °C | -27.99 | °F | ||
| Critical density | 14327 | mol/m3 | 243.99 | kg/m3 | 15.23 | lb/ft3 | 0.4734 | slug/ft3 |
| Critical pressure | 11.357 | MPa=MN/m2 | 113.57 | bar | 112.08 | atm | 1647.2 | psi=lbf/in2 |
| Critical temperature | 405.56 | K | 132.41 | °C | 270.34 | °F | ||
| Critical volume | 69.8 | cm3/mol | 0.00410 | m3/kg | 0.0657 | ft3/lb | 2.11 | ft3/slug |
| Density, gas | 41.1 | mol/m3 | 0.699 | kg/m3 | 0.0437 | lb/ft3 | 0.00136 | slug/ft3 |
| Density, liquid at -28 °F/-33.35°C, 1 atm | 40868 | mol/m3 | 696 | kg/m3 | 43.4 | lb/ft3 | 1.35 | slug/ft3 |
| Density, liquid at 70 °F/21.1°C | 36259 | mol/m3 | 617.5 | kg/m3 | 38.55 | lb/ft3 | 1.198 | slug/ft3 |
| Flammable | no | |||||||
| Flash point | 405 | K | 132 | °C | 269 | °F | ||
| Gas constant, R | 488.2 | J/kg K | 0.1356 | Wh/(kg K) | 90.74 | ft lbf/lb °R | 2919 | ft lbf/slug °R |
| Gibbs free energy of formation, ΔGf | -16.6 | kJ/mol | -975 | kJ/kg | -0.42 | Btu/lb | ||
| Heat (enthalpy) of combustion, ΔHc (gas) | 382.8 | kJ/mol | 22477 | kJ/kg | 9.663 | Btu/lb | ||
| Heat (enthalpy) of evaporation, ΔHv, at boiling point | 23.37 | kJ/mol | 1372.0 | kJ/kg | 589.87 | Btu/lb | ||
| Heat(enthalpy) of formation, ΔHf (gas) | -45.9 | kJ/mol | -2695 | kJ/kg | -1.16 | Btu/lb | ||
| Heat (enthalpy) of fusion/melting, ΔHm | 5.653 | kJ/mol | 332.3 | kJ/kg | 143 | Btu/lb | ||
| Heat (enthalpy) of sublimation, ΔHS , at 180 K | 31.2 | kJ/mol | 1832 | kJ/kg | 0.79 | Btu/lb | ||
| Ionization potential | 10.18 | eV | ||||||
| Melting (freezing) point | 195.42 | K | -77.73 | °C | -107.91 | °F | ||
| Molecular Weight | 17.03052 | g/mol | 0.03755 | lb/mol | ||||
| pH of 0.01 N aqueous solution | 10.6 | |||||||
| pH of 0.1 N aqueous solution | 11.1 | |||||||
| pH of 1.0 N aqueous solution | 11.6 | |||||||
| Standard molar entropy, S° (gas) at 1 bar | 192.77 | J/mol K | 11.32 | kJ/kg K | 0.002704 | Btu/lb °F | ||
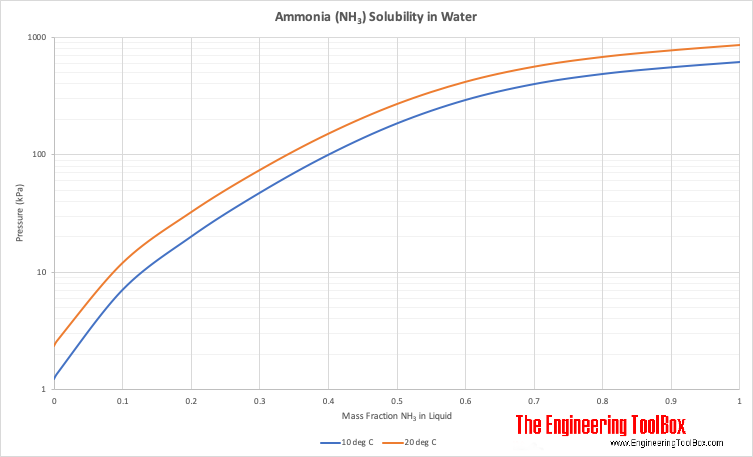
| Solubility in water, at 20°C | 540 | mg/ml | ||||||
| Solubility in water, at 24°C | 482 | mg/ml | ||||||
| Sound velocity in gas | 415 | m/s | ||||||
| Specific Gravity, gas (density relativ to air) | 0.604 | |||||||
| Specific heat (heat capacity), Cp (gas) | 37.0 | J/mol K | 2.175 | kJ/kg K | 0.5200 | Btu/lb°F = cal/g K | ||
| Specific heat (heat capacity), Cp (liquid) | 80.8 | J/mol K | 4.744 | kJ/kg K | 1.133 | Btu/lb°F = cal/g K | ||
| Specific heat ratio - Cp/Cv (gas) | 1.32 | |||||||
| Specific volume | 0.02435 | m3/mol | 1.43 | m3/kg | 22.91 | ft3/lb | 736.99 | ft3/slug |
| Surface tension at 11.1°C/52.0°F | 23.4 | dynes/cm | ||||||
| Surface tension at 34.1°C/93.4°F | 18.1 | dynes/cm | ||||||
| Thermal Conductivity | 0.026 | W/m°C | 0.015 | Btu/hr ft °F | ||||
| Triple point pressure | 0.00609 | MPa=MN/m2 | 0.0609 | bar | 0.0601 | atm | 0.883 | psi=lbf/in2 |
| Triple point temperature | 195.5 | K | -77.65 | °C | -107.77 | °F | ||
| Vapor (saturation) pressure | 1.00 | MPa=MN/m2 | 7500 | mm Hg | 9.869 | atm | 145.0 | psi=lbf/in2 |
| Vapor (saturation) pressure at -49.72°F/-45.4°C | 0.0533 | MPa=MN/m2 | 400 | mm Hg | 0.526 | atm | 7.73 | psi=lbf/in2 |
| Viscosity, dynamic (absolute) (gas) | 0.0100 | cP | 6.72 ×10-6 | lbm/ft s | 0.209 ×10-6 | lbf s/ft2 | ||
| Viscosity, dynamic (absolute) at 27°C (liq) | 0.1293 | cP | 86.89 ×10-6 | lbm/ft s | 2.70 ×10-6 | lbf s/ft2 | ||
| Viscosity, dynamic (absolute) at -33.5°C (liq) | 0.255 | cP | 171.4 ×10-6 | lbm/ft s | 5.326 ×10-6 | lbf s/ft2 |
See also the following documents for changes in ammonia properties with changes in pressure and temperature:
- Density at varying temperature and pressure
- Dynamic and Kinematic Viscosity
- Liquid Ammonia - Thermal Properties at Saturation Pressure
- Prandtl number
- Properties at Gas-Liquid Equilibrium Conditions
- Specific heat
- Thermal Conductivity at Varying Temperature and Pressure
- Vapour Pressure at gas-liquid equilibrium
See also more about atmospheric pressure, and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure,
as well as Thermophysical properties of: Acetone, Acetylene, Air, Argon, Benzene, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethanol, Ethylene, Helium, Hydrogen, Hydrogen sulfide, Methane, Methanol, Nitrogen, Oxygen, Pentane, Propane, Toluene, Water and Heavy water, D2O.
Ammonia is a gas at standard conditions. However, at low temperature and/or high pressures the gas becomes a liquid. The phase diagram for ammonia shows the phase behavior with changes in temperature and pressure. The curve between the triple point and the critical point shows the ammonia boiling point with changes in pressure.
Below the triple point temperature, ammonia becomes a solid, this phase will also be present at very high pressure (> 10 000 bar) and ambient temperature.
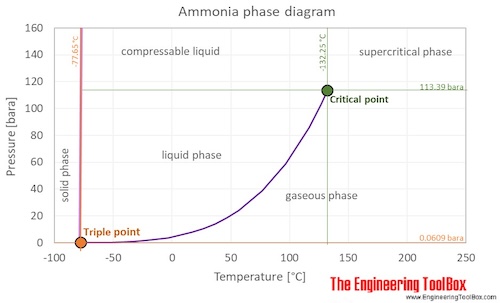
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.
Solubility of Ammonia in Water
Related Documents
Acetone - Thermophysical Properties
Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included.
Air Properties - Density, Viscosity, Heat Capacity, Thermal Conductivity, and more
Thermal properties of air, including density, viscosity, thermal conductivity, specific heat and more at different temperatures and pressures. Comprehensive reference with formulas, tables, and charts to support engineering calculations.
Ammonia - Properties at Gas-Liquid Equilibrium Conditions
Figures and tables showing how the properties of liquid and gaseous ammonia changes along the boiling/condensation curve (temperature and pressure between triple point and critical point conditions). An ammonia phase diagram are included.
Ammonia - Specific Heat vs. Temperature and Pressure
Online calculator, figures and tables showing specific heat, CP and CV, of gasous and liquid ammonia at temperatures ranging from -73 to 425°C (-100 to 800°F) at pressure ranging from 1 to 100 bara (14.5 - 1450 psia) - SI and Imperial Units.
Ammonia Gas - Density vs. Temperature and Pressure
Online calculator with figures and tables showing density and specific weight of ammonia for temperatures ranging -50 to 425 °C (-50 to 800 °F) at atmospheric and higher pressure - Imperial and SI Units.
Heavy Water - Thermophysical Properties
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more.
Methanol - Thermophysical Properties
Chemical, physical and thermal properties of methanol, CH3OH (also called carbinol, wood alcohol, hydroxy methyl and methyl alcohol). Phase diagram included.
Solubility of Gases in Water vs. Temperature
Solubility of Ammonia, Argon, Carbon Dioxide, Carbon Monoxide, Chlorine, Ethane, Ethylene, Helium, Hydrogen, Hydrogen Sulfide, Methane, Nitrogen, Oxygen and Sulfur Dioxide in water.


