Mathiasite Mineral Data (original) (raw)
General Mathiasite Information
(K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38
Molecular Weight = 1,682.56 gm
Potassium 1.39 % K 1.68 % K2O
Strontium 0.52 % Sr 0.62 % SrO
Calcium 0.71 % Ca 1.00 % CaO
Magnesium 0.72 % Mg 1.20 % MgO
Titanium 36.99 % Ti 61.72 % TiO2
Chromium 18.54 % Cr 24.25 % CrO
Iron 4.98 % Fe 6.41 % FeO
Oxygen 36.13 % O
______ ______
100.00 % 96.86 % = TOTAL OXIDE
K0.6Ca0.3Sr0.1Ti13Cr6Fe2+1.5Mg0.5O38
In kimberlite.
Approved IMA 1983
Jagersfontein mine, Orange Free State, South Africa. Link to MinDat.org Location Data.
Named for Frances Celia Morna Mathias (1913-), British-South African geochemist, University of Capetown, South Africa.
ICSD 35353
PDF 46-1468
Mathiasite Image
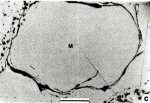 |
Mathiasite Comments: Mathiasite (M) exhibiting typical curvilinear cracks and incipient decomposition (upper left and lower right). Sample JFIS-I, Jagersfontein. Reflected light in air. (AmMin, v68:494). Location: Metasomatized peridotites and heavy media concentrates from four kimberlites in the Republic of South Africa.Scale: Scale Bar 0.2 mm. © American Mineralogist |
|---|
Mathiasite Crystallography
a:c = 1:1.99324
a = 10.36, c = 20.65, Z = 3; V = 1,919.42 Den(Calc)= 4.37
Trigonal - RhombohedralH-M Symbol ( 3) Space Group: R 3
By Intensity(I/Io): 1.44(1), 2.14(1), 2.25(0.9),
Mouse
drag1 - LMB Manipulate Structure
drag2 - RMB Resize/Rotate
Keyboard
S - Stereo Pair on/off
H - Help Screen
I - Data Info
A - Atoms On/Off
P - Polyhedra On/Off
B - Bonds On/Off
Help on Above
| | Gatehouse B M , Grey I E , Smyth J R , Acta Crystallographica, Section C , 39 (1983) p.421-422, Structure refinement of mathiasite, (K0.62Na0.14Ba0.14Sr0.10), [Ti12.90Cr3.10Mg1.53Fe2.15Zr0.67Ca0.29V0.36]O38, locality: peridotite nodules, Bultfonten kimblerlite, South africa | | --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- |
Physical Properties of Mathiasite
None
Black.
4.6
Opaque
Conchoidal - Fractures developed in brittle materials characterized by smoothly curving surfaces, (e.g. quartz).
7.5 - Garnet
Metallic
gray
Optical Properties of Mathiasite
dark tan.
weaker dark tan.
Uniaxial (?).
NCalc= 2.36 - from Gladstone-Dale relationship (KC = 0.3122) where Ncalc=Dcalc*KC+1
NCalc= 2.44 - from Gladstone-Dale relationship (KC = 0.3122) where Ncalc=Dmeas*KC+1
Moderate, brownish tan to dark brown.
Tan.
Standardized Intensity (100%) Reflection Spectra of Mathiasite in Air
| λ | R1 | R 2 |  |
∑ R1(λ) | ∑ R2(λ) |
|---|---|---|---|---|---|
| 400 nm | 20.10 | 20.80 |   |
||
| 420 nm | 19.50 | 20.20 |   |
||
| 440 nm | 19.00 | 19.70 |   |
||
| 460 nm | 18.50 | 19.20 |   |
||
| 480 nm | 18.10 | 18.60 |   |
||
| 500 nm | 17.70 | 18.40 |   |
||
| 520 nm | 17.40 | 18.20 |   |
||
| 540 nm | 17.20 | 17.90 |   |
||
| 560 nm | 16.90 | 17.70 |   |
||
| 580 nm | 16.80 | 17.60 |   |
||
| 600 nm | 16.60 | 17.40 |   |
||
| 620 nm | 16.50 | 17.40 |   |
||
| 640 nm | 16.50 | 17.30 |   |
||
| 660 nm | 16.50 | 17.30 |   |
||
| 680 nm | 16.40 | 17.30 |   |
||
| 700 nm | 16.40 | 17.30 |   |
Calculated Relative Intensity Colors of Mathiasite in Air
| RelativeIntensity | 0% | 60% | 100% | 120% | 180% | 240% | 300% | 360% | 420% | 480% | 520% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | |||||||||||
| R2 |
Calculated Properties of Mathiasite
Bulk Density (Electron Density)=4.16 gm/cc
note: Specific Gravity of Mathiasite =4.37 gm/cc.
Fermion Index = 0.06
Boson Index = 0.94
PEMathiasite = 12.77 barns/electron
U=PEMathiasite x relectron= 53.09 barns/cc.
GRapi = 19.11 (Gamma Ray American Petroleum Institute Units)
Concentration of Mathiasite per GRapi unit = 5.23 (%)
Estimated Radioactivity from Mathiasite  - barely detectable
- barely detectable
Mathiasite Classification
08.05.01.07 (08)Multiple Oxides with Nb, Ta, and Ti
(08.05)Dana Type
(08.05.01)Crichtonite group (ABC18 T2 O38)
08.05.01.01 Landauite NaMnZn2(Ti,Fe)6Ti12O38 R 3 3
08.05.01.02 Loveringite (Ca,Ce)(Ti,Fe,Cr,Mg)21O38 R 3 3
08.05.01.03 Crichtonite (Sr,La,Ce,Y)(Ti,Fe,Mn)21O38 R 3 3
08.05.01.04 Senaite Pb(Ti,Fe,Mn)21O38 R 3 3
08.05.01.05 Davidite-(La) (La,Ce,Ca)(Y,U)(Ti,Fe)20O38 R 3 3
08.05.01.06 Davidite-(Ce) (Ce,La)(Y,U)(Ti,Fe)20O38 R 3 3
08.05.01.07 Mathiasite (K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3
08.05.01.08 Lindsleyite (Ba,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3
08.05.01.09 Dessauite! (Sr,Pb)(Y,U)(Ti,Fe)20O38 P 3 3
08.05.01.10 Cleusonite! Pb(U,U)(Ti,Fe,Fe)20(O,OH)38 R 3 3
08.05.01.11 Gramaccioliite-(Y)! (Pb,Sr)(Y,Mn)Fe2(Ti,Fe)18O38 R 3 3
04.CC.40 04 - OXIDES (Hydroxides, V[5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodate
04.C - Metal:Oxygen = 2:3, 3:5, and Similar
04.CC -With large and medium-sized cations
04.CC.40 Crichtonite (Sr,La,Ce,Y)(Ti,Fe,Mn)21O38 R 3 3
04.CC.40 Dessauite! (Sr,Pb)(Y,U)(Ti,Fe)20O38 P 3 3
04.CC.40 Davidite-(Ce) (Ce,La)(Y,U)(Ti,Fe)20O38 R 3 3
04.CC.40 Davidite-(La) (La,Ce,Ca)(Y,U)(Ti,Fe)20O38 R 3 3
04.CC.40 Mathiasite (K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3
04.CC.40 Lindsleyite (Ba,Sr)(Ti,Cr,Fe,Mg)21O38 R 3 3
04.CC.40 Landauite NaMnZn2(Ti,Fe)6Ti12O38 R 3 3
04.CC.40 Loveringite (Ca,Ce)(Ti,Fe,Cr,Mg)21O38 R 3 3
04.CC.40 Senaite Pb(Ti,Fe,Mn)21O38 R 3 3
04.CC.40 Cleusonite! Pb(U,U)(Ti,Fe,Fe)20(O,OH)38 R 3 3
04.CC.40 Gramaccioliite-(Y)! (Pb,Sr)(Y,Mn)Fe2(Ti,Fe)18O38 R 3 3
Other Mathiasite Information
NAME( Dana8) PHYS. PROP.(Enc. of Minerals,2nd ed.,1990) OPTIC PROP.(AntBidBlaNic3)
Links to other databases for Mathiasite :
1 - Am. Min. Crystal Structure Database
2 - Athena
3 - EUROmin Project
4 - Ecole des Mines de Paris
5 - GeoScienceWorld
6 - Google Images
7 - Google Scholar
8 - Handbook of Mineralogy (MinSocAm)
9 - Handbook of Mineralogy (UofA)
10 - MinDAT
11 - Mineralienatlas (Deutsch)
12 - Online Mineral Museum
13 - QUT Mineral Atlas
14 - Ruff.Info
15 - WWW-MINCRYST
Search for Mathiasite using:
[AOL] [Bing] [Dog Pile] [GeoScienceWorld] [HotBot] [Ixquick] [Lycos] [MAMMA] [Scirus] [Teoma] [WebCrawler] [Wikipedia] [YAHOO]
Visit our Advertisers for Mathiasite :
A Bijoux Google Search for Mathiasite
Adam's Minerals Google Search for Mathiasite
Cape Minerals Google Search for Mathiasite
Dakota Matrix Minerals Google Search for Mathiasite
Excalibur Mineral Corp. Google Search for Mathiasite
Exceptional Minerals Google Search for Mathiasite
John Betts Fine Minerals Search for Mathiasite
McDougall Minerals Google Search for Mathiasite
Mineral News Website Link
Rock and Mineral Shows Google Search for Mathiasite
Weinrich Minerals, Inc. Google Search for Mathiasite
Ask about Mathiasite here :
Ask-A-Mineralogist from the Mineralogical Society of America
Mindat.org's Discussion Groups
Original Rockhounds Discussion Group
Rockhounds Discussion Group on Yahoo Groups
Mineral Discussion Forum from Fabre Minerals - also available in Espa�ol
Print or Cut-and-Paste your Mathiasite Specimen Label here :
Mathiasite
(K,Ca,Sr)(Ti,Cr,Fe,Mg)21O38
Dana No: 08.05.01.07 Strunz No: 04.CC.40
Locality:
Notes: