A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: The Sysdimet study (original) (raw)
Abstract
Aims/hypothesis
Low-grade inflammation and endothelial dysfunction may play a role in the pathogenesis of type 2 diabetes and cardiovascular disease. We evaluated whether a diet high in fatty fish, bilberries and wholegrain products (Healthy Diet) improves biomarkers reflecting inflammation and endothelial dysfunction in individuals with impaired glucose metabolism.
Methods
We recruited individuals with impaired glucose metabolism and features of the metabolic syndrome into a 12 week, parallel design, dietary intervention trial conducted at the Department of Clinical Nutrition, University of Eastern Finland (Kuopio, Finland). Randomisation was performed by matching according to sex and medians of age, BMI and fasting plasma glucose of the study population at screening. The primary endpoint in the present study was the change in plasma inflammatory markers and the measurements were performed blinded to group assignment. High-sensitivity (hs) C-reactive protein (CRP) and E-selectin responses were also analysed separately in participants not using statins (n = 76).
Results
Altogether, 131 individuals were assigned to either the Healthy Diet (n = 44), a whole-grain-enriched diet (WGED) (n = 42) or a control (n = 45) diet, and 104 participants (mean ± SD: age 59 ± 7 years; BMI 31.1 ± 3.5 kg/m2) who had completed the study, were analysed (Healthy Diet n = 36, WGED n = 34 and control diet n = 34). Plasma E-selectin decreased only in the Healthy Diet group. This occurred in all group participants (p < 0.05) and also after excluding participants using statins (p < 0.05). Plasma hsCRP levels decreased in the Healthy Diet (median −17%, p < 0.05) and WGED (median −27%, p < 0.01) groups in participants not using statins. Controlling for confounding factors, including BMI or insulin sensitivity, did not alter the results. A greater increase in plasma concentration of very-long-chain _n_-3 fatty acids and in the intake of fibre during the study was associated with a greater decrease in plasma E-selectin (p < 0.05). The intake of test breads consumed during the Healthy Diet and WGED interventions was inversely associated with the change in hsCRP levels (p < 0.001).
Conclusions/interpretation
Our results suggest that the combined effect of fatty fish, bilberries and wholegrain products may improve endothelial dysfunction and inflammation in overweight and obese individuals at high risk of developing diabetes.
Trial registration:
ClinicalTrials.gov NCT00573781
Funding:
The study was funded by the Academy of Finland (117844 and 118590 [to M. Uusitupa]; 131460 [to K. Poutanen]; 130469 [to H. Mykkänen] and 131593 [to V. D. F. de Mello]); the Kuopio University Hospital (5106, 5168, 5254 [to M. Uusitupa]); the Finnish Diabetes Research Foundation; the Sigrid Juselius Foundation; the Nordic Centre of Excellence on ‘Systems biology in controlled dietary interventions and cohort studies’ (SYSDIET; 070014); and the European Commission in the Communities 6th Framework Programme, Project HEALTHGRAIN (FOOD-CT-2005-514008).
Similar content being viewed by others
Introduction
Individuals with impaired glucose metabolism are at increased risk of developing type 2 diabetes [1]. Low-grade inflammation and endothelial dysfunction seem to play a role in the pathogenesis of type 2 diabetes and cardiovascular diseases (CVD) [2–6]. Epidemiological studies suggest that diets that are high in saturated fat, low in fibre, rich in carbohydrates and have a high glycaemic index may increase the risk of type 2 diabetes [7, 8].
Evidence based on cross-sectional studies, meta-analyses and systematic reviews links high consumption of whole grain to a decreased risk of metabolic syndrome, type 2 diabetes and CVD in different populations [9–12], but its effects on inflammatory markers have been less consistent [12–14]. The intake of fish has been linked to the prevention of chronic diseases involving inflammatory processes [15] and to a beneficial effect on risk factors for CVD, including circulating levels of inflammatory markers [16]. Experimental studies support a role for phenolic compounds, present in bilberries, in the prevention of inflammatory diseases including CVD [17].
There are no data in the literature about dietary changes involving the combined effects of fatty fish, bilberries and wholegrain products on inflammatory markers in individuals with impaired glucose metabolism. In this context, we evaluated whether dietary modifications based on increasing these dietary components improve selected biomarkers of inflammation and endothelial dysfunction in individuals with impaired glucose metabolism and features of the metabolic syndrome.
Methods
Participants and study design
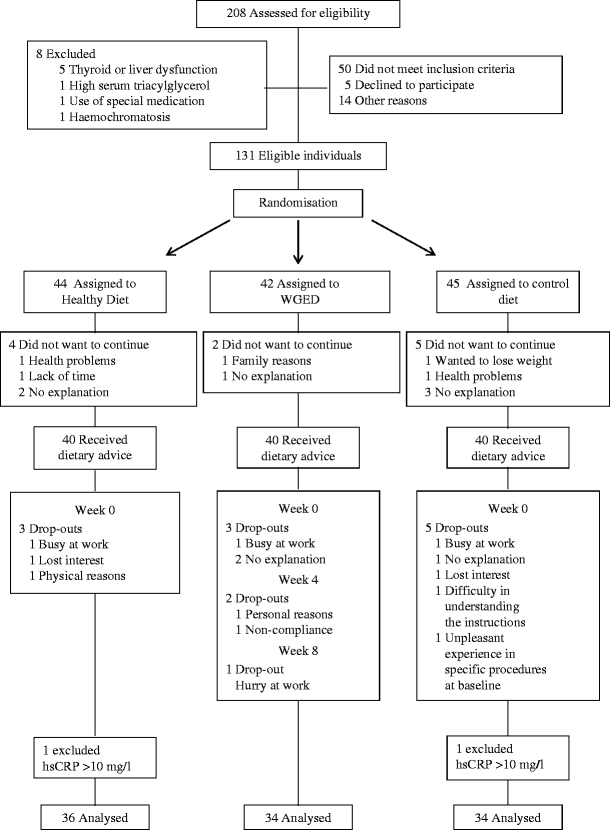
Altogether 131 participants were recruited for a 12 week parallel controlled dietary intervention study from the Kuopio area, Finland (Fig. 1). Recruitment was based on the following inclusion criteria: age 40–70 years; impaired glucose metabolism (impaired fasting glucose 5.6–6.9 mmol/l or impaired glucose tolerance 7.8–11.0 mmol/l during an OGTT [75 g glucose]); and at least two of the following: BMI 26–39 kg/m2; waist circumference ≥102 cm in men and ≥88 cm in women; serum triacylglycerol >1.7 mmol/l; HDL-cholesterol <1.0 mmol/l in men and <1.3 mmol/l in women; and blood pressure ≥130/≥85 mmHg. Individuals were excluded if they had: serum triacylglycerol >3.5 mmol/l; serum total cholesterol >8.0 mmol/l; abnormal liver, thyroid or kidney functions; a history of alcohol abuse; or were using narcoleptic or corticosteroids medication (inhaled corticosteroids allowed). Participants volunteered to the study and gave written informed consent. The study plan was approved by the Local Research Ethics Committee in accordance with the Helsinki Declaration.
Fig. 1
Flow chart of participants
The participants were randomised to a Healthy Diet, whole-grain-enriched diet (WGED) or control group. Groups were matched for sex, and for medians of BMI, age and fasting plasma glucose. Altogether 106 participants completed the study (Fig. 1). Of these, two participants, with high-sensitivity (hs) C-reactive protein (CRP) concentration >10 mg/l suggesting clinical inflammation, were excluded. Therefore, 104 individuals were analysed (Fig. 1).
Diets
In the Healthy Diet group, participants were advised to replace their usual cereal products by products with at least 50% of their composition from a wholegrain source. The breads recommended, which contributed 20% to 25% of total energy intake, were: a selection of commercial rye breads (50% of bread consumption, fibre content 10.0–14.4%), sourdough wholemeal wheat bread (10%, fibre content 6.4%) and also endosperm rye bread (40%, fibre content 6.9%). The two latter breads are known to produce a low postprandial insulin response [18–20]. The recommended intake of wholemeal pasta was at least 35 g (uncooked) per week. In addition, one daily portion of another cereal product habitually consumed by the participants was allowed. Participants were instructed to eat fatty fish [21] (100–150 g fish per meal) three times per week, and to use vegetable oil and vegetable oil-based products in fish preparation. Otherwise, we did not seek to change the quality of the dietary fat, except for an increase in fatty fish consumption. Bilberries (Vaccinium myrtillus; frozen, pureed or dried powder) were advised to be eaten as three portions per day (equivalent of 300 g fresh bilberries per day). Participants were allowed to retain one of their habitual berry portions (e.g. raspberries) three to four times per week.
In the WGED group, participants were instructed to consume the same cereal products as in the Healthy Diet group. In addition, they were given wholegrain oat snack bars [21] to be consumed once a day on a voluntary basis and were asked not to change their current fish and berry consumption.
In the control group, participants were asked to avoid wholegrain cereals. They were also instructed to replace the breads they usually consumed with refined wheat breads, and other cereal products, e.g. porridge or pasta, with low-fibre products [21]. The intake of bilberries was not allowed and consumption of fatty fish was allowed once a week only. Otherwise, the habitual diet and lifestyle habits were kept unchanged in all groups.
The cereal (bread, pasta, snack bars) and bilberry products were given free of charge during the study. The cost of buying fish was reimbursed for those in the Healthy Diet group. Participants recorded daily their consumption of the test breads (all groups), pasta (Healthy Diet and WGED), oat biscuits (WGED), bilberries (Healthy Diet) and fish (Healthy Diet). Participants in all groups kept a 4 day dietary record (consecutive days, including one weekend day) during the run-in period (baseline intake) and three times (weeks 3, 7 and 11) during the intervention period. One participant from the Healthy Diet group had no baseline dietary data, therefore the changes in dietary intake for this one participant were not included in the analyses. Dietary data were analysed using Nutrica software [22]. Body weight and height were measured at baseline, and body weight again at weeks 4, 8 and 12 (end of the study). BMI was calculated as weight (kg)/height (m2).
Biochemical analyses
Blood samples were drawn at baseline and at the end of the study (week 12), through a catheter in an antecubital vein after a 12 h overnight fast. Serum total cholesterol, LDL-cholesterol and HDL-cholesterol, and triacylglycerol were analysed using commercial kits (Thermo Electron, Vantaa, Finland). Serum insulin was analysed with a chemiluminescent immunoassay (Advia Centaur, Siemens Medical Solution Diagnostics, Tarrytown, NY, USA) and plasma glucose by the glucose hexokinase method (Konelab System Reagents; Thermo Fisher Scientific, Vantaa, Finland).
The plasma fatty acids α-linolenic acid (18:3 _n_-3, ALA), eicosapentaenoic acid (20:5 _n_-3, EPA) and docosahexaenoic acid (22:6 _n_-3, DHA) were analysed by gas chromatography [21]. High-sensitivity CRP and serum amyloid A (SAA) concentrations were determined by nephelometry (Siemens, Eschborn, Germany) with an analytical range and sensitivity of 0.175 to 11.00 mg/l and 0.16 mg/l, and 1 to 200 mg/l and 0.8 mg/l, respectively. ELISA was used to measure asymmetric dimethylarginine (DLD, Hamburg, Germany), von Willebrand factor (Matched Pair Antibody Set; Affinity Biologicals, Ancaster, ON, Canada), macrophage migration inhibitory factor (DuoSet Elisa kit; R&D Systems, Abingdon, UK), soluble intercellular cellular adhesion molecule-1 (Diaclone, Besancon, France), and E-selectin, chemokine (C-C motif) ligand 5 and IL-1 receptor antagonist (Quantikine Elisa Kits; R&D Systems). High-sensitivity ELISA kits were used to measure TNF-α and IL-6 (Quantikine and Elisa Kits; R&D Systems). Measurements were done in plasma EDTA samples, except for SAA. The inter-assay CVs for hsCRP and E-selectin were 5.0% and 7.2%, respectively. The CVs of the other markers are given in the electronic supplementary material (ESM) Table 1.
Statistical analyses
Variables with a skewed distribution were natural log-transformed before the analyses and are reported as median (interquartile range [IQR]). The effects of the dietary interventions (groups) on the relative change in plasma circulating markers were compared by applying general linear model univariate analysis and using group as a fixed factor, adjusted for age, sex and baseline measurement, followed by the Bonferroni correction for multiple comparisons. When a group effect or an overall change (intercept) was significant, Student's paired t test was used for within-group comparisons between the baseline and week 12 measurements. Because the use of statins was common in this study population (27%) and could have interfered with the effect of dietary intervention on the two inflammatory markers based on their known anti-inflammatory effects [23], we also repeated the analyses in participants not using statins (n = 76). Multivariate linear regression analyses were used to test the independent effects of the changes in dietary fibre and in plasma or dietary _n_-3 fatty acids on the changes in plasma E-selectin and hsCRP. All models used for analysing the dietary data were adjusted for total energy intake. A value of p < 0.05 was considered significant. Analyses were performed using SPSS software version 17.0 (SPSS, Chicago, IL, USA).
Results
Participant characteristics
Participants in the study groups were well matched for age, sex, body composition, blood pressure, and glucose and lipid variables (Table 1). The number of participants using statins was not different among groups (Healthy Diet n = 9, WGED n = 10, control n = 9; p = 0.92) and none of the participants stopped or started this medication during the study.
Table 1 Baseline characteristics of the participants according to study group
Diet and body composition during the study
Although at baseline the Healthy Diet group had a trend towards higher energy intake than the WGED group (p = 0.06), the change in daily energy intake was similar among the groups, even after taking the baseline energy intake into account (p = 0.18). In the within-group analyses, the reported energy intake increased in all the groups during the study as compared with baseline ([mean ± SD] Healthy Diet 8,185 ± 1,860 vs 8,980 ± 1,995 kJ/day, p = 0.003; WGED 6,995 ± 2,373 vs 7,655 ± 2,395 kJ/day, p = 0.03; control group 7,244 ± 2,028 vs 8,522 ± 1,718 kJ/day, p = 0.05).
There was a trend towards a decrease in body weight in the Healthy Diet group and towards an increase in body weight in the control group (mean ± SD: 89.8 ± 12.2 vs 89.0 ± 11.7 kg/m2, p = 0.08 and 89.5 ± 13.2 vs 89.9 ± 13.1 kg/m2, p = 0.08, respectively). These developments were also reflected in terms of BMI (mean ± SD: 31.1 ± 3.6 vs 31.0 ± 3.4 kg/m2, p = 0.08 and 30.9 ± 3.5 vs 31.0 ± 3.6 kg/m2, p = 0.05, respectively). Although the reported energy intake in the WGED group increased during the study compared with baseline, body weight and BMI did not change (89.2 ± 15.3 vs 89.1 ± 15.3 and 31.4 ± 3.4 vs 31.4 ± 3.4 kg/m2, respectively, p > 0.10 for both). However, the changes in BMI and body weight between baseline and the end of the study were not significantly different between the groups (p > 0.05 for all).
During the study, participants recorded a mean intake of test breads of 7.7, 7.9 and 6.8 portions per day in the Healthy Diet, WGED and control groups, respectively. In the WGED, the mean daily intake of the wholegrain oat snack bar was 13 g. In the Healthy Diet group, participants consumed a mean of 3.2 fish meals (>85% as fatty fish) per week; their intake of bilberries was 3.2 portions per day. The main source of fat used for preparing the fish meals was rapeseed oil. The change in dietary nutrient intake based on the 4 day food records is depicted in Table 2. While the change in carbohydrate consumption was similar among groups (p = 0.55), fibre intake increased in the Healthy Diet and WGED groups compared with the control group (p < 0.001), being even higher in the former than in the WGED group (p < 0.001).
Table 2 Nutrient intake and _n_-3 fatty acids in plasma lipids at baseline and during the study according to the study interventions and their respective relative changes
While the changes in intake of total and saturated fat were not different among groups (p > 0.05), the intake of polyunsaturated fat decreased in the WGED and control groups (p = 0.001 for both). In the Healthy Diet and WGED groups, the saturated fat intake slightly decreased during the intervention (p = 0.002 and p = 0.02, respectively). However, the magnitude of these changes was very small (Table 2). In the control group, the intake of total and saturated fat did not change (p = 0.58 and p = 0.71, respectively). In the Healthy Diet group, participants reported an increase in their intake of ALA and EPA+DHA during the study as compared with baseline (p < 0.001), thus differing from the control group (p < 0.001). In plasma, however, although the percentage of EPA+DHA (p < 0.001) and ALA (p < 0.001) increased in the Healthy Diet group, only the former was significantly different from the control (p < 0.001) and WGED (p < 0.001) groups (Table 2).
Glucose and insulin metabolism during the study
As previously found [21], although there were no statistical differences in the changes of glucose and insulin metabolism between the groups (p > 0.10 for all), the 2 h plasma glucose value decreased in the Healthy Diet (mean ± SD: 6.7 ± 1.7 vs 6.1 ± 1.7 mmol/l, p = 0.002) and WGED (6.6 ± 1.6 vs 6.1 ± 1.9 mmol/l, p = 0.009) groups; and the glucose AUC also decreased in the Healthy Diet group (mean ± SD: 244 ± 132 vs 194 ± 121 mmol/l, p = 0.007). Also in the latter we observed improvements in early-phase insulin secretion, estimated as insulinogenic index, and in the composite measure of beta cell function, estimated as the disposition index (Table 3). No change in insulin sensitivity between and within groups was observed when estimated either by fasting insulin, HOMA of insulin resistance [21], quantitative insulin sensitivity check index (QUICKI) or by the Matsuda insulin sensitivity index (Table 3).
Table 3 Indexes of insulin and glucose metabolism, and circulating levels of biomarkers related to inflammation and endothelial dysfunction before and after Healthy Diet (n = 36), WGED (n = 34) and control (n = 34) dietary interventions and their respective relative changes
Changes in plasma inflammatory markers during the study
The results of all 11 inflammation- and endothelial function-related markers are described in Table 3. Only E-selectin and hsCRP changed in different ways across the groups or after the 12 week study in within-group analyses.
Plasma concentrations of E-selectin and hsCRP did not differ among groups at baseline (p > 0.10 for both) (Table 3). Plasma E-selectin concentration in the Healthy Diet group decreased significantly during the intervention (p < 0.05), but not in the WGED or control groups (_p_ > 0.05). The change during the Healthy Diet intervention was significantly different from that in the control group, but it was not significant when compared with the WGED after testing for multiple comparisons (p = 0.02 and p = 0.26, respectively). Circulating levels of hsCRP decreased only in the WGED group (p < 0.05), but the changes among the groups were not significantly different (_p_ > 0.05) (Table 2).
In the Healthy Diet group, there was a higher proportion of individuals with high-risk hsCRP levels (>3.0 mg/l) at baseline in non-statin-users than in statin-users (33.3% vs 0%, p = 0.08). However, this difference was attenuated after the 12-week intervention (15% vs 0%, p = 0.55). These respective changes were also seen in the median hsCRP values before (non-statin 1.9 [IQR 0.8, 3.6] vs statin 1.1 [0.7, 1.4], p = 0.10) and after the intervention (1.1 [0.8, 2.6] vs 1.1 [0.9, 2.2], p = 0.91).
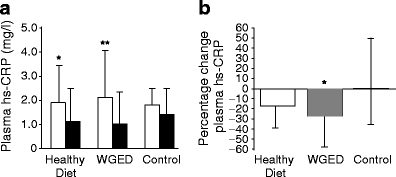
Figure 2 displays the analyses of hsCRP after excluding participants who used statins during the study. The hsCRP concentrations at baseline were not different among the groups (p = 0.91). Plasma hsCRP concentrations decreased in individuals following the WGED and Healthy Diet interventions (p < 0.01 and p < 0.05, respectively) (Fig. 2). The change in circulating levels of hsCRP in the WGED group was significantly different from that in the control group (p < 0.05). Plasma E-selectin concentrations at baseline also did not differ among groups (p = 0.55) in participants not using statins, and decreased only in the Healthy Diet group (p = 0.004). The change in E-selectin also differed between the Healthy Diet and control groups (median −9% [IQR −15, 1] vs 2% [−7, 9], p = 0.02), but not between the Healthy Diet and WGED (−2% [−7, 9], p = 0.25) groups. Adjustment for the changes in BMI, insulin sensitivity and energy intake did not alter the results. Although an increase in energy intake was associated with a decrease in hsCRP levels, a change in BMI or insulin sensitivity was not significantly associated with either of the outcomes in each respective model (data not shown).
Fig. 2
Plasma concentrations of hsCRP according to randomisation group in participants not using statins during the study. a hsCRP before (baseline; white bars) and after (week 12; black bars) in Healthy Diet (n = 27), WGED (n = 24) and control (n = 25) dietary interventions. *p < 0.05 for baseline vs week 12 after Student’s paired t test; **p < 0.01 for baseline vs week 12 after Student’s paired t test. b hsCRP relative changes ([week 12 − baseline] × 100/baseline) in Healthy Diet (n = 27), WGED (n = 24) and control (n = 25) dietary interventions. p = 0.91 for the difference among groups at baseline after one-way ANOVA; p = 0.04 for the group effect in general linear model univariate analysis; *p < 0.05 for the difference between WGED and control groups after Bonferroni correction for multiple comparisons. Values (a, b) are median and IQR
Association between changes in dietary intake and changes in plasma E-selectin and hsCRP
Additionally, we explored whether the changes in dietary intake of fibre and in dietary intake and plasma percentages of _n_-3 fatty acids could explain the change in E-selectin and hsCRP concentrations in participants not using statins (Table 4). A higher increase in the intake of fibre was significantly associated with a greater decrease in plasma E-selectin during the study. Similarly, a greater increase in the intake and plasma percentage levels of the sum of EPA and DHA was associated with a decrease in plasma E-selectin. However, the effect of the increase in ALA intake on decreasing E-selectin was not reflected by ALA plasma proportions (Table 4).
Table 4 Regression coefficients (β) for the effect of the change in the intake of fibre, and of the changes in plasma and dietary ALA and EPA + DHA on the changes in plasma E-selectin or in hsCRP in participants not using statins during the study
Interestingly, belonging to the Healthy Diet group was associated with a greater decrease in E-selectin levels compared with the control group (Fig. 2), even when including the change in intake of fibre, EPA + DHA or ALA in the models (p < 0.05 for the effect of group and for the difference between Healthy Diet and control groups in all models in participants not using statins).
In similar models, but considering hsCRP as a dependent variable, we only found a trend for an association between the increase in ALA intake and the decrease in hsCRP, and this was not confirmed at plasma levels (Table 4). Finally, and still in participants not using statins, a higher intake of test breads in the Healthy Diet and WGED interventions was strongly associated with decreased hsCRP levels (slices/day β = −0.51, p < 0.001). Of the various breads, the endosperm rye bread was the one that was highly correlated with the change in hsCRP (β = −0.59, p < 0.001), followed by the other commercial rye breads (β = −0.37, p < 0.01 for all commercial rye breads).
Discussion
Our results clearly show that a 3 month dietary intervention with an experimental diet high in fatty fish, bilberries and wholegrain products (Healthy Diet) reduced plasma E-selectin circulating levels in individuals with impaired glucose metabolism and features of the metabolic syndrome compared with a control diet that was low in fibre, fatty fish and berries, this reduction being independent of the use of lipid-lowering medication (statins) during the study. We also observed a similar decrease in hsCRP concentration in individuals on the Healthy Diet or the WGED, in the latter of which participants kept to their habitual diet, but replaced refined cereal products with wholegrain cereal products. Consistent with the main findings, greater increases in the intake of total fibre and _n_-3 fatty acids were inversely associated with greater decreases in E-selectin circulating levels.
In our study, the Healthy Diet had a beneficial impact on endothelial function as estimated by circulating levels of E-selectin. Dysfunction of the endothelium plays an integral role in atherogenesis and CVD. E-selectin, one of the molecules produced during endothelial injury and released into the circulation, has been used as a molecular marker of endothelial dysfunction [2, 24, 25]. In epidemiological and intervention studies, a benefit of _n_-3 polyunsaturated fatty acids on cardiovascular health [26, 27] and plasma E-selectin levels [28] has been suggested. A prudent diet pattern, rich in fish, whole grains and fruit, has been inversely associated with circulating levels of E-selectin and CRP [29].
We observed that the change in the intake of fibre was a determinant of the change in E-selectin levels. The total daily fibre intake increased in the WGED intervention due to the consumption of test breads. However, the intake of fibre in the Healthy Diet was even higher due to the consumption not only of the test breads, but also of bilberries [22], which likely contributed to the observed reduction of plasma E-selectin and the association between plasma E-selectin and fibre intake in this group. On the other hand, fibre intake alone may not explain the results. Bilberries (from the Vaccinium family), for example, are particularly abundant in polyphenols, and anthocyanins account for most of the bilberry total polyphenol content [30]. Bilberry anthocyanins have been shown to exert a wide range of biological effects, including antioxidant and vasodilatory actions [17]. Moreover, these anthocyanins and other phenolic compounds also found in bilberries may provide substantial antioxidant protection [31], which could benefit endothelial function and decrease CVD risk [17, 32].
Our results suggest that the increase in dietary and plasma _n_-3 fatty acids plays a role in the increase in E-selectin levels. However, data from intervention trials on the beneficial effects of fish intake on surrogate markers of endothelial function are controversial [33]. The source of EPA + DHA may also be important, as fish-based diets have been proposed to have a more beneficial effect than fish oil supplements on circulating inflammatory markers [34]. Although a greater increase in the intake of ALA was associated with an increase in E-selectin levels, this association was not reflected at the plasma level, so this result must be treated with caution.
Overall, the Healthy Diet and WGED interventions had an anti-inflammatory effect as estimated by within-group changes in hsCRP. A Mediterranean-style diet, in which whole grains and fibre are highly consumed, has been shown to ameliorate circulating levels of CRP and to reduce the risk of type 2 diabetes mellitus [35, 36]. Fish intake and _n_-3 fatty acids have been shown to decrease CRP levels [37], albeit not in all studies [38, 39]. Based on our study design, we hypothesised that there would be a greater decrease in this inflammatory marker with the Healthy Diet than with the WGED. However, we did not find any significant association between the changes in dietary _n_-3 fatty acids and circulating levels of hsCRP during the study. Moreover, data on the beneficial effect of bilberry intake on hsCRP levels are lacking, suggesting that other nutrients or phytochemical compounds could also have contributed to the decrease in CRP levels.
One of the most interesting findings in our study is that a higher intake of test breads (90% of which were rye breads) by participants in the Healthy Diet and WGED interventions was associated with a greater decrease in hsCRP levels. Previous work conducted by our colleagues has shown that rye breads, regardless of fibre content, have beneficial effects on insulin metabolism by lowering postprandial insulin response and increasing early-phase insulin secretion [19, 20]. Hypothetically, increased early postprandial hyperinsulinaemia can also be pro-inflammatory [40]. In this context, it has also been shown that consumption of a diet (rye bread and pasta) geared to low insulin response might have anti-inflammatory properties [41].
Other factors, such as changes in body weight, insulin sensitivity, saturated fat and energy intake, could also have affected the decreases observed in inflammatory and endothelial markers after the dietary interventions [37, 42–45]. However, in multivariate analyses taking into account the minor changes in BMI or the recorded saturated fat or energy intakes during the study the results were not altered with respect to E-selectin and hsCRP. Furthermore, our results suggest that insulin sensitivity also does not explain the beneficial effects of the dietary interventions on these inflammatory markers.
We selected individuals at increased risk of CVD and type 2 diabetes for this study [46, 47]. Elevated circulating levels of E-selectin, among other markers of endothelial dysfunction, and high hsCRP levels have been found to predict type 2 diabetes mellitus [4–6, 24] and CVD [3, 25, 48]. Moreover, lifestyle interventions involving dietary changes, which are able to reduce the risk of diabetes, have also improved low-grade inflammation as measured by CRP [36, 43]. Therefore, the anti-inflammatory effect of the intervention diets in the present study may be important in the long-term prevention of type 2 diabetes mellitus and CVD.
Our observations also hint at a potential role of the Healthy Diet in lowering hsCRP close to the levels seen in statin users, as also previously shown when statin therapy was compared with cholesterol-lowering diets [49]. Taking this together with the beneficial effect of the above diet on E-selectin concentrations, it seems possible that the potential anti-atherogenic effect of a Healthy Diet could be through other mechanisms than the lowering of LDL-cholesterol, including, for example, HDL-cholesterol metabolism, and anti-inflammatory and antioxidant properties [50].
In this study, the individuals from the control group decreased their fibre intake by replacing their usually consumed breads with refined wheat breads and by limiting their consumption of bilberries. Considering the normal eating habits in Eastern Finland, where consumption of rye bread and berries is common, these dietary instructions needed to be given in order to determine the effects of the experimental diets. The high intake of fibre at baseline in the Healthy Diet group could also have biased our findings. However, adjustments for this variable in the models did not alter the main findings on the changes in E-selectin and hsCRP levels after the interventions.
In conclusion, a diet high in fatty fish, bilberries and wholegrain products may decrease inflammation and endothelial dysfunction as measured by hsCRP and E-selectin, an effect that occurs independently of insulin sensitivity, in overweight and obese individuals at high risk of developing type 2 diabetes and CVD.
Abbreviations
ALA:
α-Linolenic acid
CRP:
C-reactive protein
CVD:
Cardiovascular diseases
DHA:
Docosahexaenoic acid
EPA:
Eicosapentaenoic acid
hs:
High-sensitivity
IQR:
Interquartile range
SAA:
Serum amyloid A
WGED:
Whole-grain-enriched diet
References
- Genuth S, Alberti KG, Bennett P et al (2003) Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26:3160–3167
Article PubMed Google Scholar - Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Article PubMed CAS Google Scholar - Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G et al (2010) C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375:132–140
Article PubMed Google Scholar - Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Article PubMed CAS Google Scholar - Laaksonen DE, Niskanen L, Nyyssonen K et al (2004) C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia 47:1403–1410
Article PubMed CAS Google Scholar - Meigs JB, O'Donnell CJ, Tofler GH et al (2006) Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes 55:530–537
Article PubMed CAS Google Scholar - Hu FB, van Dam RM, Liu S (2001) Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 44:805–817
Article PubMed CAS Google Scholar - Lindström J, Peltonen M, Eriksson JG et al (2006) High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: the Finnish Diabetes Prevention Study. Diabetologia 49:912–920
Article PubMed Google Scholar - Sahyoun NR, Jacques PF, Zhang XL, Juan W, McKeown NM (2006) Whole-grain intake is inversely associated with the metabolic syndrome and mortality in older adults. Am J Clin Nutr 83:124–131
PubMed CAS Google Scholar - Mellen PB, Walsh TF, Herrington DM (2008) Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis 18:283–290
Article PubMed Google Scholar - Priebe MG, van Binsbergen JJ, de Vos R, Vonk RJ (2008) Whole grain foods for the prevention of type 2 diabetes mellitus. Cochrane Database Syst Rev, Issue 1, Art. no: CD006061. doi:10.1002/14651858.CD006061.pub2
- Jensen MK, Koh-Banerjee P, Franz M, Sampson L, Gronbaek M, Rimm EB (2006) Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J Clin Nutr 83:275–283
PubMed CAS Google Scholar - Masters RC, Liese AD, Haffner SM, Wagenknecht LE, Hanley AJ (2010) Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J Nutr 140:587–594
Article PubMed CAS Google Scholar - Brownlee IA, Moore C, Chatfield M et al (2010) Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br J Nutr 104:125–134
Article PubMed CAS Google Scholar - Wall R, Ross RP, Fitzgerald GF, Stanton C (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 68:280–289
Article PubMed Google Scholar - Smith KM, Barraj LM, Kantor M, Sahyoun NR (2009) Relationship between fish intake, _n_-3 fatty acids, mercury and risk markers of CHD (National Health and Nutrition Examination Survey 1999–2002). Public Health Nutr 12:1261–1269
Article PubMed Google Scholar - Basu A, Rhone M, Lyons TJ (2010) Berries: emerging impact on cardiovascular health. Nutr Rev 68:168–177
Article PubMed Google Scholar - Lappi J, Selinheimo E, Schwab U et al (2010) Sourdough fermentation of wholemeal wheat bread increases solubility of arabinoxylan and protein and decreases postprandial glucose and insulin responses. J Cereal Sci 51:152–158
Article CAS Google Scholar - Juntunen KS, Laaksonen DE, Autio K et al (2003) Structural differences between rye and wheat breads but not total fiber content may explain the lower postprandial insulin response to rye bread. Am J Clin Nutr 78:957–964
PubMed CAS Google Scholar - Juntunen KS, Laaksonen DE, Poutanen KS, Niskanen LK, Mykkanen HM (2003) High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am J Clin Nutr 77:385–391
PubMed CAS Google Scholar - Lankinen M, Schwab US, Kolehmainen M et al. (2011) Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the Sysdimet Study. PLoS ONE (in press)
- National Institute for Health and Welfare, Nutrition Unit (2010) Fineli: Finnish food composition database. Release 11. National Institute for Health and Welfare, Helsinki
- Ridker PM, Danielson E, Fonseca FA et al (2009) Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet 373:1175–1182
Article PubMed CAS Google Scholar - Meigs JB, Hu FB, Rifai N, Manson JE (2004) Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA 291:1978–1986
Article PubMed CAS Google Scholar - Hwang SJ, Ballantyne CM, Sharrett AR et al (1997) Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 96:4219–4225
PubMed CAS Google Scholar - Erkkilä A, de Mello VDF, Risérus U, Laaksonen DE (2008) Dietary fatty acids and cardiovascular disease: an epidemiological approach. Prog Lipid Res 47:172–187
Article PubMed Google Scholar - Adkins Y, Kelley DS (2010) Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 21:781–792
Article PubMed CAS Google Scholar - Thies F, Miles EA, Nebe-von-Caron G et al (2001) Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 36:1183–1193
Article PubMed CAS Google Scholar - Lopez-Garcia E, Schulze MB, Fung TT et al (2004) Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 80:1029–1035
PubMed CAS Google Scholar - Määttä-Riihinen KR, Kamal-Eldin A, Mattila PH, Gonzalez-Paramás AM, Törrönen AR (2004) Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J Agric Food Chem 52:4477–4486
Article PubMed Google Scholar - Määttä-Riihinen KR, Kähkönen MP, Törrönen AR, Heinonen IM (2005) Catechins and procyanidins in berries of vaccinium species and their antioxidant activity. J Agric Food Chem 53:8485–8491
Article PubMed Google Scholar - Münzel T, Gori T, Bruno RM, Taddei S (2010) Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J 31:2741–2748
Article PubMed Google Scholar - Egert S, Stehle P (2011) Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care 14:121–131
Article PubMed CAS Google Scholar - Myhrstad MC, Retterstol K, Telle-Hansen VH et al (2011) Effect of marine n-3 fatty acids on circulating inflammatory markers in healthy subjects and subjects with cardiovascular risk factors. Inflamm Res 60:309–319
Article PubMed CAS Google Scholar - Salas-Salvadó J, Garcia-Arellano A, Estruch R et al (2008) Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr 62:651–659
Article PubMed Google Scholar - Salas-Salvadó J, Bullo M, Babio N et al (2011) Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 34:14–19
Article PubMed Google Scholar - Tsitouras PD, Gucciardo F, Salbe AD, Heward C, Harman SM (2008) High omega-3 fat intake improves insulin sensitivity and reduces CRP and IL6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res 40:199–205
Article PubMed CAS Google Scholar - Madsen T, Christensen JH, Blom M, Schmidt EB (2003) The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br J Nutr 89:517–522
Article PubMed CAS Google Scholar - Damsgaard CT, Frokiaer H, Andersen AD, Lauritzen L (2008) Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr 138:1061–1066
PubMed CAS Google Scholar - Ludwig DS (2002) The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287:2414–2423
Article PubMed CAS Google Scholar - Kallio P, Kolehmainen M, Laaksonen DE et al (2008) Inflammation markers are modulated by responses to diets differing in postprandial insulin responses in individuals with the metabolic syndrome. Am J Clin Nutr 87:1497–1503
PubMed CAS Google Scholar - de Mello VD, Kolehmainen M, Schwab U et al (2008) Effect of weight loss on cytokine messenger RNA expression in peripheral blood mononuclear cells of obese subjects with the metabolic syndrome. Metabolism 57:192–199
Article PubMed Google Scholar - Herder C, Peltonen M, Koenig W et al (2009) Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia 52:433–442
Article PubMed CAS Google Scholar - Petersson H, Lind L, Hulthe J, Elmgren A, Cederholm T, Riserus U (2009) Relationships between serum fatty acid composition and multiple markers of inflammation and endothelial function in an elderly population. Atherosclerosis 203:298–303
Article PubMed CAS Google Scholar - Festa A, Hanley AJ, Tracy RP, D'Agostino R Jr, Haffner SM (2003) Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation 108:1822–1830
Article PubMed CAS Google Scholar - Lakka HM, Laaksonen DE, Lakka TA et al (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288:2709–2716
Article PubMed Google Scholar - Meigs JB (2010) Epidemiology of type 2 diabetes and cardiovascular disease: translation from population to prevention: the Kelly West award lecture 2009. Diabetes Care 33:1865–1871
Article PubMed Google Scholar - Bonetti PO, Lerman LO, Lerman A (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23:168–175
Article PubMed CAS Google Scholar - Jenkins DJ, Kendall CW, Marchie A et al (2003) Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA 290:502–510
Article PubMed CAS Google Scholar - Haas MJ, Mooradian AD (2011) Inflammation, high-density lipoprotein and cardiovascular dysfunction. Curr Opin Infect Dis 24:265–272
Article PubMed CAS Google Scholar - Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470
Article PubMed CAS Google Scholar
Acknowledgements
This study was supported by: the Academy of Finland (117844 and 118590 [to M. Uusitupa]; 131460 [to K. Poutanen]; 130469 [to H. Mykkänen] and 131593 [to V. D. F. de Mello]); the Kuopio University Hospital (5106, 5168, 5254 [to M. Uusitupa]); the Finnish Diabetes Research Foundation; the Sigrid Juselius Foundation; the Nordic Centre of Excellence on ‘Systems biology in controlled dietary interventions and cohort studies’ (SYSDIET; 070014); and the European Commission in the Communities 6th Framework Programme, Project HEALTHGRAIN (FOOD-CT-2005-514008). We thank the laboratory technologists T. Onnukka and K. Kettunen from the Department of Clinical Nutrition, University of Eastern Finland, Kuopio, Finland, and G. Trischler from the Department of Internal Medicine II-Cardiology, University of Ulm Medical Center, Ulm, Germany for their skilful work.
Contribution statement
US, MK, MU, KP and HM designed the study; WK and MS were responsible for the inflammatory data analyses and interpretation; VDFdeM was responsible for the statistical analyses and interpretation of the data. VDFdeM, US and MU wrote the manuscript. All the authors reviewed the manuscript critically and approved the final version.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
- Department of Clinical Nutrition/Food and Health Research Centre, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio Campus, P.O. Box 1627, FIN-70211, Kuopio, Finland
V. D. F. de Mello, U. Schwab, M. Kolehmainen, M. Siloaho, K. Poutanen, H. Mykkänen & M. Uusitupa - Institute of Clinical Medicine, Internal Medicine, Kuopio University Hospital, Kuopio, Finland
U. Schwab - Department of Internal Medicine II-Cardiology, University of Ulm Medical Center, Ulm, Germany
W. Koenig - Institute of Clinical Medicine, Clinical Research Centre, University of Eastern Finland, Kuopio, Finland
M. Siloaho - VTT, Technical Research Centre of Finland, Espoo, Finland
K. Poutanen - Research Unit, Kuopio University Hospital, Kuopio, Finland
M. Uusitupa
Authors
- V. D. F. de Mello
You can also search for this author inPubMed Google Scholar - U. Schwab
You can also search for this author inPubMed Google Scholar - M. Kolehmainen
You can also search for this author inPubMed Google Scholar - W. Koenig
You can also search for this author inPubMed Google Scholar - M. Siloaho
You can also search for this author inPubMed Google Scholar - K. Poutanen
You can also search for this author inPubMed Google Scholar - H. Mykkänen
You can also search for this author inPubMed Google Scholar - M. Uusitupa
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toV. D. F. de Mello.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Mello, V.D.F., Schwab, U., Kolehmainen, M. et al. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: The Sysdimet study.Diabetologia 54, 2755–2767 (2011). https://doi.org/10.1007/s00125-011-2285-3
- Received: 10 May 2011
- Accepted: 25 July 2011
- Published: 26 August 2011
- Issue Date: November 2011
- DOI: https://doi.org/10.1007/s00125-011-2285-3