Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation (original) (raw)
Abstract
Aims/hypothesis
The aim of this work was to assess the effect of liraglutide on ectopic fat accumulation in individuals with type 2 diabetes mellitus.
Methods
This study is a pre-specified subanalysis of the MAGNetic resonance Assessment of VICTOza efficacy in the Regression of cardiovascular dysfunction In type 2 diAbetes mellitus (MAGNA VICTORIA) study, with primary endpoints being the effects of liraglutide on left ventricular diastolic and systolic function. The MAGNA VICTORIA study was a single-centre, parallel-group trial in 50 individuals with type 2 diabetes mellitus (BMI >25 kg/m2) who were randomly assigned (1:1, stratified for sex and insulin use) to receive liraglutide 1.8 mg once daily or placebo for 26 weeks, added to standard care. Participants, study personnel and outcome assessors were blinded to treatment allocation. The secondary endpoints of visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (SAT) and epicardial fat were measured with MRI. Hepatic triacylglycerol content (HTGC) and myocardial triacylglycerol content (MTGC) were quantified with proton MR spectroscopy. Between-group differences (change from baseline) were tested for significance using ANCOVA. Mean differences with 95% CIs were reported.
Results
The trial was completed in 2016. Twenty-four participants were randomised to receive liraglutide and 26 to receive placebo. One patient in the liraglutide group withdrew consent before having received the study drug and was not included in the intention-to-treat analysis. Liraglutide (n = 23) vs placebo (n = 26) significantly reduced body weight (liraglutide 98.4 ± 13.8 kg to 94.3 ± 14.9 kg; placebo 94.5 ± 13.1 kg to 93.9 ± 13.2 kg; estimated treatment effect −4.5 [95% CI −6.4, −2.6] kg). HbA1c declined in both groups without a significant treatment effect of liraglutide vs placebo (liraglutide 66.7 ± 11.5 mmol/mol to 55.0 ± 13.2 mmol/mol [8.4 ± 1.1% to 7.3 ± 1.2%]; placebo 64.7 ± 10.2 mmol/mol to 56.9 ± 6.9 mmol/mol [8.2 ± 1.0% to 7.5 ± 0.7%]; estimated treatment effect −2.9 [95% CI −8.1, 2.3] mmol/mol or −0.3 [95% CI −0.8, 0.2]%). VAT did not change significantly between groups (liraglutide 207 ± 87 cm2 to 203 ± 88 cm2; placebo 204 ± 63 cm2 to 200 ± 55 cm2; estimated treatment effect −7 [95% CI −24, 10] cm2), while SAT was reduced by a significantly greater extent with liraglutide than with placebo (liraglutide 361 ± 142 cm2 to 339 ± 131 cm2; placebo 329 ± 107 cm2 to 333 ± 125 cm2; estimated treatment effect −29 [95% CI −51, −8] cm2). Epicardial fat did not change significantly between groups (liraglutide 8.9 ± 4.3 cm2 to 9.1 ± 4.7 cm2; placebo 9.6 ± 4.1 cm2 to 9.6 ± 4.6 cm2; estimated treatment effect 0.2 [95% CI −1.5, 1.8] cm2). Change in HTGC was not different between groups (liraglutide 18.1 ± 11.2% to 12.0 ± 7.7%; placebo 18.4 ± 9.4% to 14.7 ± 10.0%; estimated treatment effect −2.1 [95% CI −5.3, 1.0]%). MTGC was not different after treatment with liraglutide (1.5 ± 0.6% to 1.2 ± 0.6%) vs placebo (1.3 ± 0.5% to 1.2 ± 0.6%), with an estimated treatment effect of −0.1 (95% CI −0.4, 0.2)%. There were no adjudicated serious adverse events.
Conclusions/interpretation
Compared with placebo, liraglutide-treated participants lost significantly more body weight. Liraglutide primarily reduced subcutaneous fat but not visceral, hepatic, myocardial or epicardial fat. Future larger studies are needed to confirm the results of this secondary endpoint study.
Trial registration
ClinicalTrials.gov NCT01761318.
Funding
This study was funded by Novo Nordisk A/S (Bagsvaerd, Denmark).
Similar content being viewed by others
Introduction
Insulin resistance and type 2 diabetes mellitus are hallmarked by excess fat storage in visceral adipose tissue (VAT), liver, skeletal muscle, myocardial tissue and epicardial fat [[1](/article/10.1007/s00125-019-05021-6#ref-CR1 "van der Meer RW, Lamb HJ, Smit JW, de Roos A (2012) MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology 264(1):21–37. https://doi.org/10.1148/radiol.12110772
")]. VAT is tightly linked to insulin resistance and cardiovascular disease, independent of general obesity [[1](/article/10.1007/s00125-019-05021-6#ref-CR1 "van der Meer RW, Lamb HJ, Smit JW, de Roos A (2012) MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology 264(1):21–37. https://doi.org/10.1148/radiol.12110772
")]. Consequently, diet-induced reduction of VAT has greater impact on markers of insulin sensitivity and cardiometabolic risk than reduction of subcutaneous adipose tissue (SAT) [[2](/article/10.1007/s00125-019-05021-6#ref-CR2 "Park HS, Lee K (2005) Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: a study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet Med 22(3):266–272. https://doi.org/10.1111/j.1464-5491.2004.01395.x
")]. Hepatic steatosis is an independent predictor of cardiovascular disease, possibly by contributing to hepatic insulin resistance resulting in atherogenic dyslipidaemia [[1](/article/10.1007/s00125-019-05021-6#ref-CR1 "van der Meer RW, Lamb HJ, Smit JW, de Roos A (2012) MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology 264(1):21–37. https://doi.org/10.1148/radiol.12110772
")]. Excess hepatic fat accumulation can damage the liver itself when uncomplicated steatosis progresses to non-alcoholic steatohepatitis (NASH). In parallel, myocardial steatosis and excess epicardial fat are postulated to negatively affect myocardial function and coronary vasculature, respectively [[3](/article/10.1007/s00125-019-05021-6#ref-CR3 "Levelt E, Mahmod M, Piechnik SK et al (2016) Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65(1):44–52. https://doi.org/10.2337/db15-0627
")]. Therefore, therapeutic interventions aimed at reducing excess ectopic fat storage might have a major impact on the cardiovascular prognosis of individuals with type 2 diabetes mellitus.
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist (GLP-1RA) commonly used to achieve glycaemic control in individuals with type 2 diabetes. Liraglutide’s actions include stimulation of glucose-dependent insulin release from beta cells, and promotion of satiety resulting in reduced energy intake and modest weight loss. Since modest weight loss, at least by energy restriction, is associated with a reduction in ectopic fat accumulation [[4](/article/10.1007/s00125-019-05021-6#ref-CR4 "Chaston TB, Dixon JB (2008) Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 32(4):619–628. https://doi.org/10.1038/sj.ijo.0803761
")], liraglutide might also have this effect. In addition, it has been shown in animal models that endogenous GLP-1 or GLP-1RAs exert pleiotropic actions in organs such as the liver and heart, and in animal studies it has been found that GLP-1 receptor (GLP-1R) agonism can decrease hepatic [[5](#ref-CR5 "Ben-Shlomo S, Zvibel I, Shnell M et al (2011) Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 54(6):1214–1223. https://doi.org/10.1016/j.jhep.2010.09.032
"),[6](#ref-CR6 "Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA (2011) GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One 6(9):e25269. https://doi.org/10.1371/journal.pone.0025269
"),[7](/article/10.1007/s00125-019-05021-6#ref-CR7 "Parlevliet ET, Wang Y, Geerling JJ et al (2012) GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS One 7(11):e49152. https://doi.org/10.1371/journal.pone.0049152
")] and myocardial [[8](/article/10.1007/s00125-019-05021-6#ref-CR8 "Noyan-Ashraf MH, Shikatani EA, Schuiki I et al (2013) A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127(1):74–85. https://doi.org/10.1161/circulationaha.112.091215
")] steatosis, independent of weight loss. In humans, studies with GLP-1RAs that assessed VAT and hepatic steatosis in individuals with type 2 diabetes have shown conflicting results, possibly related to use of different GLP-1RAs, treatment differences in control arms and varying baseline participant characteristics [[9](#ref-CR9 "Bi Y, Zhang B, Xu W et al (2014) Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol 51(5):865–873. https://doi.org/10.1007/s00592-014-0638-3
"),[10](#ref-CR10 "Jendle J, Nauck MA, Matthews DR et al (2009) Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 11(12):1163–1172. https://doi.org/10.1111/j.1463-1326.2009.01158.x
"),[11](#ref-CR11 "Li CJ, Yu Q, Yu P et al (2014) Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol 13:36. https://doi.org/10.1186/1475-2840-13-36
"),[12](#ref-CR12 "Santilli F, Simeone PG, Guagnano MT et al (2017) Effects of liraglutide on weight loss, fat distribution, and beta-cell function in obese subjects with prediabetes or early type 2 diabetes. Diabetes Care 40(11):1556–1564. https://doi.org/10.2337/dc17-0589
"),[13](#ref-CR13 "Shi L, Zhu J, Yang P et al (2017) Comparison of exenatide and acarbose on intra-abdominal fat content in patients with obesity and type-2 diabetes: a randomized controlled trial. Obes Res Clin Pract 11(5):607–615. https://doi.org/10.1016/j.orcp.2017.01.003
"),14,[15](/article/10.1007/s00125-019-05021-6#ref-CR15 "Tang A, Rabasa-Lhoret R, Castel H et al (2015) Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: a randomized trial. Diabetes Care 38(7):1339–1346. https://doi.org/10.2337/dc14-2548
")]. Furthermore, it has been shown that response to intervention may vary between different ectopic fat depots [[16](/article/10.1007/s00125-019-05021-6#ref-CR16 "Jonker JT, de Mol P, de Vries ST et al (2013) Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology 269(2):434–442. https://doi.org/10.1148/radiol.13121631
")]. Therefore, an integrated assessment on a multi-organ level is required to fully establish the potency of liraglutide in combatting ectopic fat and in long-term patient outcome. Probably due to technical challenges, myocardial steatosis and epicardial fat have been incorporated as read-outs in only few studies [[17](/article/10.1007/s00125-019-05021-6#ref-CR17 "Dutour A, Abdesselam I, Ancel P et al (2016) Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 18(9):882–891. https://doi.org/10.1111/dom.12680
"), [18](/article/10.1007/s00125-019-05021-6#ref-CR18 "Iacobellis G, Mohseni M, Bianco SD, Banga PK (2017) Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) 25(2):311–316. https://doi.org/10.1002/oby.21718
")].
Recent technical developments have enabled non-invasive direct quantification of ectopic fat depots in humans with high accuracy using MRI and proton magnetic resonance spectroscopy (1H-MRS) [[19](/article/10.1007/s00125-019-05021-6#ref-CR19 "Bizino MB, Sala ML, de Heer P et al (2015) MR of multi-organ involvement in the metabolic syndrome. Magn Reson Imaging Clin N Am 23(1):41–58. https://doi.org/10.1016/j.mric.2014.09.010
")]. Using these techniques, we assessed ectopic fat as a pre-specified secondary study of the previously published MAGNetic resonance Assessment of VICTO2a efficacy in the Regression of cardiovascular dysfunction In type 2 diAbetes mellitus (MAGNA VICTORIA) study [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")]. The primary purpose of that randomised placebo-controlled study was to evaluate the effect of liraglutide on left ventricular diastolic and systolic function in 50 individuals with type 2 diabetes. In the liraglutide group, early diastolic filling, stroke volume and ejection fraction reduced, compared with the placebo group. In keeping with the relationship between ectopic fat and cardiac function described above, the aim of the present study was to evaluate whether liraglutide reduces visceral fat, hepatic steatosis and myocardial steatosis.
Methods
Study design and participants
This study was part of the MAGNA VICTORIA study, which was an investigator-initiated randomised, double-blind, assessor-blinded, placebo-controlled, single-centre clinical trial with 26 weeks follow-up [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")]. Electronic supplementary material (ESM) Table 1 provides an overview of the ClinicalTrials.gov registered trial endpoints that have already been published, those reported in the present manuscript, and endpoints that will be published in future manuscripts. In short, the study aimed to include 50 participants (men and women) aged 18–69 years with BMI 25 kg/m2 or above and HbA1c level of 53–86 mmol/mol (7.0–10.0%) despite use of metformin, and/or sulfonylurea derivative (SUD) and/or insulin. Main exclusion criteria were as follows: use of other glucose-lowering therapy; renal, hepatic or cardiovascular disease; gastric bypass surgery; chronic pancreatitis or previous acute pancreatitis; pregnancy or lactation; and MRI contra-indications. The trial was approved by the local ethics committee and performed in accordance with the principles of the revised Declaration of Helsinki. Written informed consent was obtained from all participants before study. The trial was conducted at the Leiden University Medical Center (LUMC), Leiden, the Netherlands and was registered at ClinicalTrials.gov (registration no. NCT01761318).
Randomisation and treatment
Included participants were randomised (1:1, stratification for sex and insulin use) to receive liraglutide (Victoza; Novo Nordisk, Bagsvaerd, Denmark) or placebo (provided by Novo Nordisk). The study drug was up-titrated to 1.8 mg once daily from week 3 onwards. The dose was reduced if necessitated by adverse events. During the study, blood-glucose-lowering drugs were titrated according to clinical practice guidelines by means of dose adjustment of SUD and/or insulin.
Blood examinations
At study entry and at 26 weeks, blood examinations were performed after participants had fasted for at least 6 h. HbA1c was measured using boronate affinity high-performance liquid chromatography (Primus Ultra; Siemens Healthcare Diagnostics, Breda, the Netherlands) throughout the first part of the study, and changed to measurement using ion-exchange high-performance liquid chromatography (Tosoh G8; Sysmex Nederland, Etten-Leur, the Netherlands) for subsequent measurements. HbA1c values assessed by the boronate affinity method were corrected on the basis of the correlation coefficient derived from a validation experiment that used data of 196 samples measured on both analysers. All other blood samples were processed and analysed as described previously [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")]. Adiponectin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase and γ-glutamyl transferase (GGT) concentrations were measured with a Modular P800 analyser (Roche Diagnostics, Mannheim, Germany). Serum NEFA were measured using the NEFA C kit (Wako Diagnostics, INstruchemie, Delfzijl, the Netherlands).
MRI protocol
All participants underwent an MRI and 1H-MRS protocol using a clinical 3 Tesla Ingenia whole-body MR system (Philips Medical Systems, Best, the Netherlands) at baseline and follow-up. Participants were scanned in the supine position after they had fasted for at least 6 h. The body coil was used for transmission, and reception was achieved with a 16-element anterior array and 12-element posterior array. A 3D breath-hold dual-echo mDIXON sequence of the abdomen was performed (repetition time 3.5 ms; first echo time 1.19 ms; second echo time 2.3 ms; flip angle 10°; spatial resolution 16 × 17 mm; slice thickness 4 mm; slice gap 2 mm) with transverse slice orientation. Using MASS software (LUMC, Leiden, the Netherlands), post-processing involved generation of three 10 mm transverse slices with 2 mm slice gap at the level of the fourth and fifth lumbar vertebral bodies. Semi-automated segmentation of VAT and abdominal SAT was depicted by threshold-based inclusion of fat, with manual correction. VAT and SAT were calculated as mean area of fat in three slices. 1H-MRS of the liver was performed using a 20 × 20 × 20 mm voxel of interest, which was localised using a Point Resolved Spectroscopy Sequence (echo time 35 ms; repetition time 9 s for unsuppressed spectra and 3.5 s for water-suppressed spectra). The voxel was placed preferably in liver segment V, VI, VII or VIII. The position of the voxel at baseline was used to guide placement of the voxel at follow-up MRI in the same liver segment. Four signal averages were acquired without, and 32 with, water suppression using the Multiply Optimized Insensitive Suppression Train sequence. Spectra were acquired during free-breathing at end-expiration with pencil beam navigator-based respiratory triggering [[21](/article/10.1007/s00125-019-05021-6#ref-CR21 "de Heer P, Bizino MB, Lamb HJ, Webb AG (2016) Parameter optimization for reproducible cardiac (1) H-MR spectroscopy at 3 Tesla. J Magn Reson Imaging 44(5):1151–1158. https://doi.org/10.1002/jmri.25254
")]. 1H-MRS of the heart was assessed as described previously [[21](/article/10.1007/s00125-019-05021-6#ref-CR21 "de Heer P, Bizino MB, Lamb HJ, Webb AG (2016) Parameter optimization for reproducible cardiac (1) H-MR spectroscopy at 3 Tesla. J Magn Reson Imaging 44(5):1151–1158. https://doi.org/10.1002/jmri.25254
")]. The 15 × 25 × 40 mm voxel of interest was placed in the interventricular myocardial septum. Six signal averages were acquired without, and 48 with, water suppression. Otherwise, acquisition was the same as described above. Post-processing of proton spectra was performed with an in-house developed program as previously described [[21](/article/10.1007/s00125-019-05021-6#ref-CR21 "de Heer P, Bizino MB, Lamb HJ, Webb AG (2016) Parameter optimization for reproducible cardiac (1) H-MR spectroscopy at 3 Tesla. J Magn Reson Imaging 44(5):1151–1158. https://doi.org/10.1002/jmri.25254
")], before fitting the spectra in the time-domain using the Java-based MR User Interface (version 5.0; Katholieke Universiteit Leuven, Leuven, Belgium). An ECG-gated breath-hold high-resolution water-suppressed Black-Blood Turbo Spin Echo Sequence (repetition time 1000 ms; echo time 11 ms; flip angle 90°; voxel 1.09 × 1.12 mm) in four-chamber view at end-diastole was used to image epicardial and paracardial fat. Epicardial fat was defined as the inner layer directly covering the myocardial outer surface and paracardial fat as the outer layer of fat surrounding the heart. The atrioventricular plane was set to determine the basal border. Pericardial fat was defined as the sum of epicardial and paracardial fat. All images and proton spectra were assessor blinded.
Study endpoints
We previously reported the primary endpoints of the MAGNA VICTORIA study that involved left ventricular diastolic and systolic function [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")]. The study endpoints VAT, SAT, hepatic triacylglycerol content (HTGC), myocardial triacylglycerol content (MTGC) and epicardial fat reported in the current manuscript were secondary endpoints of the MAGNA VICTORIA study. Other pre-specified endpoints were body weight, BMI, waist:hip ratio, HbA1c, serum triacylglycerols, NEFA, total cholesterol, HDL-cholesterol, LDL-cholesterol, adiponectin and liver enzymes. Endpoints that were not predefined were paracardial and pericardial fat.
Statistics
Data are shown as mean ± SD when normally distributed, or as median (interquartile range) when not normally distributed. For all presented study endpoints, we performed an ANCOVA of between-group differences of change from baseline, with randomisation arm as fixed effect and baseline measurement of dependent variable as covariate. In addition, a sensitivity analysis was performed that also included sex and insulin use as covariates in the ANCOVA model, since randomisation was stratified by sex and insulin use. Mean changes from baseline ± SD are reported for within group changes, and estimated treatment effect with 95% CIs are displayed for between-group differences. In addition to these pre-specified analyses, an assessment of associations between observed change in HTGC and change in HbA1c was performed. First, correlation between change in HbA1c and HTGC was estimated using Pearson’s correlation. Subsequently, a stepwise multiple linear regression analysis was performed to assess the following: (1) unadjusted association of the independent variables change in HbA1c, sex, age, treatment group allocation and weight loss with the dependent variable change in HTGC; (2) adjusted association for the independent variables mentioned above. All statistical analyses were performed as described previously [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")]. We considered a p value of <0.05 statistically significant.
Results
Participants were enrolled between December 2013 and September 2015, with the final participant visit taking place in March 2016. The trial flow chart was published previously [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")]. One participant in the liraglutide group withdrew consent before having received the study drug and was not included in intention-to-treat analysis, and one participant was withdrawn from the study because of frequent hypoglycaemic events (on further examination, a diagnosis of type 1 diabetes was made). In the placebo group, one participant was lost to follow-up. Intention-to-treat analysis was performed in 23 participants in the liraglutide group and 26 in placebo group. Baseline characteristics are shown in Table 1. Sex, insulin use, age, lipid levels, smoking history and glycaemic control were comparable between groups. Liraglutide recipients had slightly higher BMI. During the study, SUD and insulin doses were titrated on ambulant glucose levels and HbA1c values. This resulted in decreased total use of SUDs and insulin in liraglutide-treated participants and an increase in placebo-treated participants. An overview of concomitant drug use was described previously [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")].
Table 1 Baseline characteristics of trial population
Anthropometric measurements and laboratory values
Changes in anthropometric and laboratory measures are displayed in Table 2. Liraglutide significantly decreased body weight compared with placebo (liraglutide 98.4 ± 13.8 kg to 94.3 ± 14.9 kg; placebo 94.5 ± 13.1 kg to 93.9 ± 13.2 kg). In addition, both waist and hip circumference reduced in liraglutide- vs placebo-treated participants, without a difference in waist:hip ratio. There was no difference between groups for any of the laboratory measures (see Table 2). HbA1c differences from baseline were not different between groups. In liraglutide-treated participants, HbA1c decreased from 66.7 ± 11.5 mmol/mol to 55.0 ± 13.2 mmol/mol (8.4 ± 1.1% to 7.3 ± 1.2%), and in placebo-treated participants HbA1c decreased from 64.7 ± 10.2 mmol/mol to 56.9 ± 6.9 mmol/mol (8.2 ± 1.0% to 7.5 ± 0.7%).
Table 2 Within-group and between-group changes from baseline of anthropometric and laboratory measurements
Ectopic fat
The results of the MRI and MRS analysis of ectopic fat are summarised in Table 3. ESM Table 2 shows the sensitivity analysis with randomisation stratifiers sex and insulin use as additional covariates. The result of this analysis was similar to that of the primary analysis shown in Table 3. Liraglutide did not reduce VAT compared with placebo. In contrast, liraglutide significantly reduced SAT compared with placebo. The between-group changes in the VAT:SAT ratio were not different (estimated mean treatment effect 0.03 [95% CI −0.09, 0.04], p = 0.43). 1H-MRS of the liver was technically not successful on two occasions (one baseline measurement; one follow-up measurement in a different participant), and one participant in the placebo group displayed a biologically implausible rise in HTGC from 1.7% at baseline to 39.5% at follow-up without changes in serum liver enzymes. This measurement was therefore excluded from analysis (sensitivity analysis revealed no differences after exclusion of this measurement, data not shown). 1H-MRS of the heart was successful on all but four occasions: one at baseline due to low signal-to-noise ratio and three at follow-up due to low signal-to-noise ratio or incorrect peak frequency. With regard to MTGC, there was also no significant treatment effect of liraglutide vs placebo. With regard to epicardial, paracardial and pericardial fat, between-group differences were not significant. None of the ectopic fat variables showed significant correlation with indices of left ventricular function that had been published previously [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")] (data not shown).
Table 3 Ectopic fat accumulation
Association between HbA1c and hepatic steatosis
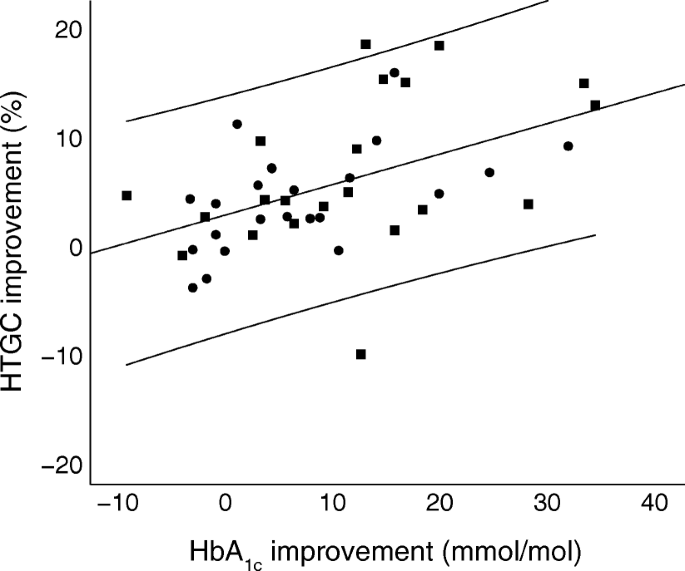
Reduction of HbA1c correlated well with reduction of HTGC in the whole cohort (r = 0.49, p = 0.001). Figure 1 shows a scatterplot of the change in HbA1c and change in HTGC between baseline and follow-up. The regression line had an unadjusted slope of 0.28 (95% CI 0.12, 0.44, p = 0.001). Stepwise multiple linear regression analysis revealed that, of the independent variables change in HbA1c, sex, age, treatment group allocation and weight loss, only change in HbA1c significantly correlated with change in HTGC (ESM Table 3). After adjustment for sex, age, treatment group allocation and weight loss, the adjusted estimate of association for change in HbA1c was 0.50 (p = 0.001).
Fig. 1
Scatter plot of HbA1c vs HTGC difference (baseline – follow-up at 26 weeks) for all participants. Circles, placebo; squares, liraglutide. The unstandardised regression line (y = 2.57 + 0.28_x_) with 95% CI is shown
Discussion
This study shows that, compared with placebo, liraglutide reduced body weight and subcutaneous fat but not visceral fat, hepatic steatosis, myocardial steatosis, epicardial fat, paracardial fat or pericardial fat. Despite a significant 4 kg weight loss in liraglutide-treated participants over 6 months, there was no reduction of ectopic fat accumulation.
In addition to blood-glucose lowering, liraglutide decreases energy intake and lowers body weight. In view of the preferential loss of VAT by modest weight loss induced by diet [[4](/article/10.1007/s00125-019-05021-6#ref-CR4 "Chaston TB, Dixon JB (2008) Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 32(4):619–628. https://doi.org/10.1038/sj.ijo.0803761
")], one would expect that liraglutide-associated weight loss would also diminish VAT. In this study, however, both waist:hip ratio and MRI assessment of abdominal fat are consistent with a preferential loss of SAT. While this result is in line with the findings of Suzuki et al., who used a dose of 0.9 mg liraglutide daily in a single-arm intervention study [14], others have shown reduction of predominantly VAT by GLP-1RA treatment [[9](/article/10.1007/s00125-019-05021-6#ref-CR9 "Bi Y, Zhang B, Xu W et al (2014) Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol 51(5):865–873. https://doi.org/10.1007/s00592-014-0638-3
"), [11](#ref-CR11 "Li CJ, Yu Q, Yu P et al (2014) Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol 13:36. https://doi.org/10.1186/1475-2840-13-36
"),[12](#ref-CR12 "Santilli F, Simeone PG, Guagnano MT et al (2017) Effects of liraglutide on weight loss, fat distribution, and beta-cell function in obese subjects with prediabetes or early type 2 diabetes. Diabetes Care 40(11):1556–1564. https://doi.org/10.2337/dc17-0589
"),[13](/article/10.1007/s00125-019-05021-6#ref-CR13 "Shi L, Zhu J, Yang P et al (2017) Comparison of exenatide and acarbose on intra-abdominal fat content in patients with obesity and type-2 diabetes: a randomized controlled trial. Obes Res Clin Pract 11(5):607–615. https://doi.org/10.1016/j.orcp.2017.01.003
"), [22](/article/10.1007/s00125-019-05021-6#ref-CR22 "Pastel E, McCulloch LJ, Ward R et al (2017) GLP-1 analogue-induced weight loss does not improve obesity-induced AT dysfunction. Clin Sci (Lond) 131(5):343–353. https://doi.org/10.1042/cs20160803
")] or no effect on SAT or VAT [[10](/article/10.1007/s00125-019-05021-6#ref-CR10 "Jendle J, Nauck MA, Matthews DR et al (2009) Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 11(12):1163–1172. https://doi.org/10.1111/j.1463-1326.2009.01158.x
")]. These studies were performed in type 2 diabetes patients with varying ethnicity, BMI and concomitant treatment regimes, making it difficult to compare results.
It is interesting to speculate on the mechanism by which liraglutide could preferentially diminish SAT. First, it can be hypothesised that liraglutide directly affects the lipogenesis:lipolysis ratio in adipose tissue by binding to GLP-1Rs in adipocytes. However, studies using validated specific methods have not been able to detect the canonical GLP-1R in adipocytes [[23](/article/10.1007/s00125-019-05021-6#ref-CR23 "Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756. https://doi.org/10.1016/j.cmet.2018.03.001
")], so other mechanisms are more likely. Probably, the abundance of GLP-1Rs in white adipose tissue arises from expression in non-adipocyte cells, such as vascular endothelial cells, peripheral neurons and/or macrophages, which have been shown to express GLP1-Rs [[23](/article/10.1007/s00125-019-05021-6#ref-CR23 "Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756. https://doi.org/10.1016/j.cmet.2018.03.001
"), [24](/article/10.1007/s00125-019-05021-6#ref-CR24 "Lee YS, Park MS, Choung JS et al (2012) Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 55(9):2456–2468. https://doi.org/10.1007/s00125-012-2592-3
")]. In mice, treatment with GLP-1 affects white adipose tissue macrophage phenotype, inflammatory cytokine profile, lipogenic gene expression and fat oxidation in conjunction with increased insulin sensitivity [[24](/article/10.1007/s00125-019-05021-6#ref-CR24 "Lee YS, Park MS, Choung JS et al (2012) Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 55(9):2456–2468. https://doi.org/10.1007/s00125-012-2592-3
")]. Although these mouse studies provide a theoretical basis for a direct effect of GLP-1RAs on white adipose tissue, it is not known whether these mechanisms play a role in body fat distribution in humans. Another potential mechanism by which liraglutide could influence body fat distribution is the central nervous system. The autonomic nervous system has been shown to provide distinct innervation of VAT and SAT [[25](/article/10.1007/s00125-019-05021-6#ref-CR25 "Kreier F, Kap YS, Mettenleiter TC et al (2006) Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 147(3):1140–1147. https://doi.org/10.1210/en.2005-0667
")], each having its own sympathetic and parasympathetic innervations with catabolic and anabolic effects, respectively [[26](/article/10.1007/s00125-019-05021-6#ref-CR26 "Kreier F, Fliers E, Voshol PJ et al (2002) Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest 110(9):1243–1250. https://doi.org/10.1172/jci15736
")]. These findings, in combination with the knowledge that isolated central nervous system administration of GLP-1RAs can reduce body fat in mice with diet-induced obesity [[27](/article/10.1007/s00125-019-05021-6#ref-CR27 "Kooijman S, Wang Y, Parlevliet ET et al (2015) Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 58(11):2637–2646. https://doi.org/10.1007/s00125-015-3727-0
")], provide a theoretical basis for the hypothesis that liraglutide can alter body fat distribution via distinct action on sympathetic and parasympathetic stimulation with consequent lipolytic and lipogenic action, respectively.
Targeting hepatic steatosis is an important part of type 2 diabetes mellitus management. When considering the lack of beneficial effect of liraglutide vs placebo in our study participants, one must also consider that, in addition to placebo, concomitant SUD and insulin were titrated to reach the HbA1c goal <53 mmol/mol (7%). Therefore, our study is best compared with studies using active comparators against GLP-1RA treatment. In their open-label trial, Tang et al. treated individuals with type 2 diabetes with liraglutide vs insulin, resulting in comparable improvement in glycaemic control among treatment arms, and no between-group difference in liver fat fraction [[15](/article/10.1007/s00125-019-05021-6#ref-CR15 "Tang A, Rabasa-Lhoret R, Castel H et al (2015) Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: a randomized trial. Diabetes Care 38(7):1339–1346. https://doi.org/10.2337/dc14-2548
")]. Bi et al. reported corresponding results in their open-label randomised trial with exenatide vs insulin [[9](/article/10.1007/s00125-019-05021-6#ref-CR9 "Bi Y, Zhang B, Xu W et al (2014) Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol 51(5):865–873. https://doi.org/10.1007/s00592-014-0638-3
")]. In contrast to our study, several studies have shown that treatment with a GLP-1RA reduced HTGC when compared with treatment with insulin [[17](/article/10.1007/s00125-019-05021-6#ref-CR17 "Dutour A, Abdesselam I, Ancel P et al (2016) Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 18(9):882–891. https://doi.org/10.1111/dom.12680
"), [28](/article/10.1007/s00125-019-05021-6#ref-CR28 "Yan J, Yao B, Kuang H et al (2018) Liraglutide, sitagliptin and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and NAFLD. Hepatology (Baltimore, Md). https://doi.org/10.1002/hep.30320
")]. Dutour et al. performed a randomised open-label study in which individuals with type 2 diabetes were treated with exenatide vs insulin and were assessed for HTGC after a standard meal and showed a 23.8 ± 9.5% relative reduction in HTGC for the exenatide group compared with a 12.5 ± 9.6% increase in the insulin group (p = 0.007) [[17](/article/10.1007/s00125-019-05021-6#ref-CR17 "Dutour A, Abdesselam I, Ancel P et al (2016) Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 18(9):882–891. https://doi.org/10.1111/dom.12680
")]. Yan et al. also found HTGC to be significantly decreased in liraglutide-treated but not insulin-treated individuals [[28](/article/10.1007/s00125-019-05021-6#ref-CR28 "Yan J, Yao B, Kuang H et al (2018) Liraglutide, sitagliptin and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and NAFLD. Hepatology (Baltimore, Md). https://doi.org/10.1002/hep.30320
")]. Although body weight and HbA1c reductions in these studies were comparable with those reported in our study, an important difference was that individuals already using insulin were excluded in these studies. Whether that explains the discrepancy with our study is unclear because randomised studies comparing the effect of add-on GLP-1RA on liver fat in insulin-treated individuals are currently lacking. In a single-arm study by Petit et al., 68 individuals with type 2 diabetes (21% using insulin at baseline) were treated with liraglutide 1.2 mg once daily, resulting in a 31% RR reduction of hepatic steatosis [[29](/article/10.1007/s00125-019-05021-6#ref-CR29 "Petit JM, Cercueil JP, Loffroy R et al (2017) Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the Lira-NAFLD Study. J Clin Endocrinol Metab 102(2):407–415. https://doi.org/10.1210/jc.2016-2775
")]. This cohort was compared against another study cohort with comparable baseline characteristics (n = 16) who underwent intensification of the glucose-lowering regimen with insulin and who showed a non-significant decline of liver fat fraction. Apart from the non-randomised design, the results of this study could have been influenced by the fact that patients also received instructions on healthy diet and exercise. The only study evaluating the effect of liraglutide on histological resolution of NASH was the Liraglutide Safety and Efficacy in Patients with Non-alcoholic steatohepatitis (LEAN) trial [[30](/article/10.1007/s00125-019-05021-6#ref-CR30 "Armstrong MJ, Gaunt P, Aithal GP et al (2016) Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387(10019):679–690. https://doi.org/10.1016/s0140-6736(15)00803-x
")]. This placebo-controlled randomised trial in 52 individuals with biopsy-proven NASH showed that liraglutide led to histological resolution of NASH. In this trial, however, only 33% of participants had type 2 diabetes and there was no active comparator, resulting in a significant improvement of HbA1c in the liraglutide vs control group. In keeping with the hypothesis that reduction in HbA1c is associated with reduction in HTGC, we and others [[9](/article/10.1007/s00125-019-05021-6#ref-CR9 "Bi Y, Zhang B, Xu W et al (2014) Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol 51(5):865–873. https://doi.org/10.1007/s00592-014-0638-3
"), [15](/article/10.1007/s00125-019-05021-6#ref-CR15 "Tang A, Rabasa-Lhoret R, Castel H et al (2015) Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: a randomized trial. Diabetes Care 38(7):1339–1346. https://doi.org/10.2337/dc14-2548
"), [31](/article/10.1007/s00125-019-05021-6#ref-CR31 "Cuthbertson DJ, Irwin A, Gardner CJ et al (2012) Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One 7(12):e50117. https://doi.org/10.1371/journal.pone.0050117
")] have found a significant correlation between HbA1c reduction and HTGC reduction. One theory could be that improved glycaemic control directly reduced HTGC (e.g. by decreased de novo lipogenesis via carbohydrate response element binding protein [[32](/article/10.1007/s00125-019-05021-6#ref-CR32 "Ishii S, Iizuka K, Miller BC, Uyeda K (2004) Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci U S A 101(44):15597–15602. https://doi.org/10.1073/pnas.0405238101
")]). However, in light of conflicting results in clinical studies, more research is needed to provide insight on this topic.
Myocardial steatosis is characterised by an increased triacylglycerol content in cardiomyocytes, as assessed by 1H-MRS of the heart. The abundance of intracellular triacylglycerols is associated with increased deposition of toxic lipids that interfere with cardiac energy metabolism and cell survival [[19](/article/10.1007/s00125-019-05021-6#ref-CR19 "Bizino MB, Sala ML, de Heer P et al (2015) MR of multi-organ involvement in the metabolic syndrome. Magn Reson Imaging Clin N Am 23(1):41–58. https://doi.org/10.1016/j.mric.2014.09.010
")]. In individuals with type 2 diabetes, myocardial steatosis is a predictor of concentric left ventricular remodelling, impaired systolic strain and diastolic dysfunction [[3](/article/10.1007/s00125-019-05021-6#ref-CR3 "Levelt E, Mahmod M, Piechnik SK et al (2016) Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65(1):44–52. https://doi.org/10.2337/db15-0627
"), [33](/article/10.1007/s00125-019-05021-6#ref-CR33 "Rijzewijk LJ, van der Meer RW, Smit JW et al (2008) Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52(22):1793–1799. https://doi.org/10.1016/j.jacc.2008.07.062
")]. Reversal of myocardial steatosis with very-low-energy diet is associated with improved left ventricular diastolic function [[34](/article/10.1007/s00125-019-05021-6#ref-CR34 "Hammer S, Snel M, Lamb HJ et al (2008) Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 52(12):1006–1012. https://doi.org/10.1016/j.jacc.2008.04.068
")]. Based on these observations, and preclinical studies showing improved cardiac lipotoxicity in GLP-1RA-treated mice [[8](/article/10.1007/s00125-019-05021-6#ref-CR8 "Noyan-Ashraf MH, Shikatani EA, Schuiki I et al (2013) A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127(1):74–85. https://doi.org/10.1161/circulationaha.112.091215
"), [35](/article/10.1007/s00125-019-05021-6#ref-CR35 "Wu L, Wang K, Wang W et al (2018) Glucagon-like peptide-1 ameliorates cardiac lipotoxicity in diabetic cardiomyopathy via the PPARalpha pathway. Aging Cell:e12763. https://doi.org/10.1111/acel.12763
")] and in vitro protection from ceramide-induced cardiomyocyte apoptosis by GLP-1RA [[36](/article/10.1007/s00125-019-05021-6#ref-CR36 "Leonardini A, D’Oria R, Incalza MA et al (2017) GLP-1 Receptor Activation Inhibits Palmitate-Induced Apoptosis via Ceramide in Human Cardiac Progenitor Cells. J Clin Endocrinol Metab 102(11):4136–4147. https://doi.org/10.1210/jc.2017-00970
")], liraglutide might have the potential to reverse myocardial steatosis in individuals with type 2 diabetes. Conversely, our data do not support this hypothesis. This is also in conjunction with the fact that liraglutide did not ameliorate left ventricular myocardial relaxation (i.e. diastolic function) in this particular study population, and that left ventricular systolic function slightly decreased, presumably in relation to decreased left ventricular preload [[20](/article/10.1007/s00125-019-05021-6#ref-CR20 "Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
")]. These results are in keeping with another study performed in this particular research area [[17](/article/10.1007/s00125-019-05021-6#ref-CR17 "Dutour A, Abdesselam I, Ancel P et al (2016) Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 18(9):882–891. https://doi.org/10.1111/dom.12680
")].
Excess epicardial fat accumulation is hypothesised to exert local effects by leading to secretion of inflammatory and metabolic peptides by tissues that might contribute to coronary artery disease. Epicardial adiposity has been linked to visceral obesity [[37](/article/10.1007/s00125-019-05021-6#ref-CR37 "Levelt E, Pavlides M, Banerjee R et al (2016) Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol 68(1):53–63. https://doi.org/10.1016/j.jacc.2016.03.597
")] and coronary events [[38](/article/10.1007/s00125-019-05021-6#ref-CR38 "Mahabadi AA, Berg MH, Lehmann N et al (2013) Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 61(13):1388–1395. https://doi.org/10.1016/j.jacc.2012.11.062
")] and is a reversible phenomenon upon weight loss [[39](/article/10.1007/s00125-019-05021-6#ref-CR39 "van Eyk HJ, van Schinkel LD, Kantae V et al (2018) Caloric restriction lowers endocannabinoid tonus and improves cardiac function in type 2 diabetes. Nutr Diabetes 8(1):6–10. https://doi.org/10.1038/s41387-017-0016-7
")]. However, there was no reduction in epicardial fat volume in liraglutide-treated participants in this study. As suggested by their shared origin, it might be that epicardial and visceral fat have responded likewise to liraglutide treatment [[40](/article/10.1007/s00125-019-05021-6#ref-CR40 "Chau YY, Bandiera R, Serrels A et al (2014) Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16(4):367–375. https://doi.org/10.1038/ncb2922
")]. Iacobellis et al. and Dutour et al. did find a significant reduction in epicardial fat [[17](/article/10.1007/s00125-019-05021-6#ref-CR17 "Dutour A, Abdesselam I, Ancel P et al (2016) Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 18(9):882–891. https://doi.org/10.1111/dom.12680
"), [18](/article/10.1007/s00125-019-05021-6#ref-CR18 "Iacobellis G, Mohseni M, Bianco SD, Banga PK (2017) Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) 25(2):311–316. https://doi.org/10.1002/oby.21718
")] in their open-label studies. A possible explanation for this discrepancy could be the larger weight loss in these studies, which may have been based on their non-blinded study design.
Liraglutide has been shown to have a safe cardiovascular endpoint profile, with less major cardiovascular events compared with placebo added to standard care [[41](/article/10.1007/s00125-019-05021-6#ref-CR41 "Marso SP, Daniels GH, Brown-Frandsen K et al (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. https://doi.org/10.1056/NEJMoa1603827
")]. There are many hypotheses on the mechanisms by which GLP-1RAs might reduce cardiovascular risk, including lowering of blood pressure, improved vascular endothelial function, improved lipid metabolism, reduced inflammatory profile, direct beneficial effect on the heart, and weight loss [[23](/article/10.1007/s00125-019-05021-6#ref-CR23 "Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756. https://doi.org/10.1016/j.cmet.2018.03.001
")]. Our finding that liraglutide does not reduce ectopic fat supports the hypothesis that weight loss is not the main driver of the cardiovascular benefit of GLP-1RA therapy.
The primary limitation of this study was that the presented outcome measures were not the primary outcomes of the MAGNA VICTORIA study. This could imply that the study was underpowered with regard to evaluation of the treatment effect on ectopic fat. In that regard, the 95% CIs of between-group changes from baseline are crucial to the interpretation of this study [42]. In line with the fact that 5% mean weight loss difference between liraglutide and placebo-treated participants closely approximates the average weight loss use of liraglutide 1.8 mg in larger studies [[43](/article/10.1007/s00125-019-05021-6#ref-CR43 "Davies MJ, Bergenstal R, Bode B et al (2015) Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Jama 314(7):687–699. https://doi.org/10.1001/jama.2015.9676
")], it is likely that the ectopic fat changes are also representative. Obviously, given the heterogeneity of our study population, we cannot exclude the possibility that certain subgroups of individuals (e.g. those with severe hepatic steatosis) might benefit from liraglutide therapy with respect to lowering hepatic steatosis.
In conclusion, this pre-specified secondary study showed that liraglutide did not reduce ectopic fat accumulation in individuals with type 2 diabetes, compared with placebo treatment added to standard care. Tight glycaemic control in both treatment groups was associated with reduced hepatic steatosis, with no added effect of liraglutide. From a clinical perspective, weight loss caused by liraglutide therapy might not be crucial for its beneficial cardiovascular actions, wherein other mechanisms such as inflammation and lipid metabolism are probably involved [[23](/article/10.1007/s00125-019-05021-6#ref-CR23 "Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756. https://doi.org/10.1016/j.cmet.2018.03.001
")]. Future studies are desirable to explore whether ectopic fat accumulation can be reduced with GLP-1RAs in certain subgroups, such as those with BMI >35 kg/m2 and/or more severe hepatic steatosis.
Data availability
Data are available upon request to the guarantors of the work M.B. Bizino or H.J. Lamb.
Abbreviations
1H-MRS:
Proton magnetic resonance spectroscopy
ALT:
Alanine aminotransferase
AST:
Aspartate aminotransferase
GGT:
γ-Glutamyl transferase
GLP-1:
Glucagon-like peptide-1
GLP-1R:
GLP-1 receptor
GLP-1RA:
GLP-1 receptor agonist
HTGC:
Hepatic triacylglycerol content
MAGNA VICTORIA:
MAGNetic resonance Assessment of VICTOza efficacy in the Regression of cardiovascular dysfunction In type 2 diAbetes mellitus
MTGC:
Myocardial triacylglycerol content
NASH:
Non-alcoholic steatohepatitis
SAT:
Subcutaneous adipose tissue
SUD:
Sulfonylurea derivative
VAT:
Visceral adipose tissue
References
- van der Meer RW, Lamb HJ, Smit JW, de Roos A (2012) MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology 264(1):21–37. https://doi.org/10.1148/radiol.12110772
Article PubMed Google Scholar - Park HS, Lee K (2005) Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: a study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet Med 22(3):266–272. https://doi.org/10.1111/j.1464-5491.2004.01395.x
Article CAS PubMed Google Scholar - Levelt E, Mahmod M, Piechnik SK et al (2016) Relationship between left ventricular structural and metabolic remodeling in type 2 diabetes. Diabetes 65(1):44–52. https://doi.org/10.2337/db15-0627
Article CAS PubMed Google Scholar - Chaston TB, Dixon JB (2008) Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 32(4):619–628. https://doi.org/10.1038/sj.ijo.0803761
Article CAS Google Scholar - Ben-Shlomo S, Zvibel I, Shnell M et al (2011) Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 54(6):1214–1223. https://doi.org/10.1016/j.jhep.2010.09.032
Article CAS PubMed Google Scholar - Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA (2011) GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One 6(9):e25269. https://doi.org/10.1371/journal.pone.0025269
Article CAS PubMed PubMed Central Google Scholar - Parlevliet ET, Wang Y, Geerling JJ et al (2012) GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS One 7(11):e49152. https://doi.org/10.1371/journal.pone.0049152
Article CAS PubMed PubMed Central Google Scholar - Noyan-Ashraf MH, Shikatani EA, Schuiki I et al (2013) A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127(1):74–85. https://doi.org/10.1161/circulationaha.112.091215
Article CAS PubMed Google Scholar - Bi Y, Zhang B, Xu W et al (2014) Effects of exenatide, insulin, and pioglitazone on liver fat content and body fat distributions in drug-naive subjects with type 2 diabetes. Acta Diabetol 51(5):865–873. https://doi.org/10.1007/s00592-014-0638-3
Article CAS PubMed Google Scholar - Jendle J, Nauck MA, Matthews DR et al (2009) Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 11(12):1163–1172. https://doi.org/10.1111/j.1463-1326.2009.01158.x
Article CAS PubMed Google Scholar - Li CJ, Yu Q, Yu P et al (2014) Changes in liraglutide-induced body composition are related to modifications in plasma cardiac natriuretic peptides levels in obese type 2 diabetic patients. Cardiovasc Diabetol 13:36. https://doi.org/10.1186/1475-2840-13-36
Article CAS PubMed PubMed Central Google Scholar - Santilli F, Simeone PG, Guagnano MT et al (2017) Effects of liraglutide on weight loss, fat distribution, and beta-cell function in obese subjects with prediabetes or early type 2 diabetes. Diabetes Care 40(11):1556–1564. https://doi.org/10.2337/dc17-0589
Article CAS PubMed Google Scholar - Shi L, Zhu J, Yang P et al (2017) Comparison of exenatide and acarbose on intra-abdominal fat content in patients with obesity and type-2 diabetes: a randomized controlled trial. Obes Res Clin Pract 11(5):607–615. https://doi.org/10.1016/j.orcp.2017.01.003
Article Google Scholar - Suzuki D, Toyoda M, Kimura M et al (2013) Effects of liraglutide, a human glucagon-like peptide-1 analogue, on body weight, body fat area and body fat-related markers in patients with type 2 diabetes mellitus. Intern Med 52(10):1029–1034
Article CAS PubMed Google Scholar - Tang A, Rabasa-Lhoret R, Castel H et al (2015) Effects of insulin glargine and liraglutide therapy on liver fat as measured by magnetic resonance in patients with type 2 diabetes: a randomized trial. Diabetes Care 38(7):1339–1346. https://doi.org/10.2337/dc14-2548
Article CAS PubMed Google Scholar - Jonker JT, de Mol P, de Vries ST et al (2013) Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology 269(2):434–442. https://doi.org/10.1148/radiol.13121631
Article PubMed Google Scholar - Dutour A, Abdesselam I, Ancel P et al (2016) Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab 18(9):882–891. https://doi.org/10.1111/dom.12680
Article CAS PubMed Google Scholar - Iacobellis G, Mohseni M, Bianco SD, Banga PK (2017) Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) 25(2):311–316. https://doi.org/10.1002/oby.21718
Article CAS Google Scholar - Bizino MB, Sala ML, de Heer P et al (2015) MR of multi-organ involvement in the metabolic syndrome. Magn Reson Imaging Clin N Am 23(1):41–58. https://doi.org/10.1016/j.mric.2014.09.010
Article PubMed Google Scholar - Bizino MB, Jazet IM, Westenberg JJM et al (2019) Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol 18(1):55–12. https://doi.org/10.1186/s12933-019-0857-6
Article CAS PubMed PubMed Central Google Scholar - de Heer P, Bizino MB, Lamb HJ, Webb AG (2016) Parameter optimization for reproducible cardiac (1) H-MR spectroscopy at 3 Tesla. J Magn Reson Imaging 44(5):1151–1158. https://doi.org/10.1002/jmri.25254
Article PubMed Google Scholar - Pastel E, McCulloch LJ, Ward R et al (2017) GLP-1 analogue-induced weight loss does not improve obesity-induced AT dysfunction. Clin Sci (Lond) 131(5):343–353. https://doi.org/10.1042/cs20160803
Article CAS Google Scholar - Drucker DJ (2018) Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab 27(4):740–756. https://doi.org/10.1016/j.cmet.2018.03.001
Article CAS PubMed Google Scholar - Lee YS, Park MS, Choung JS et al (2012) Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 55(9):2456–2468. https://doi.org/10.1007/s00125-012-2592-3
Article CAS PubMed Google Scholar - Kreier F, Kap YS, Mettenleiter TC et al (2006) Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 147(3):1140–1147. https://doi.org/10.1210/en.2005-0667
Article CAS PubMed Google Scholar - Kreier F, Fliers E, Voshol PJ et al (2002) Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest 110(9):1243–1250. https://doi.org/10.1172/jci15736
Article CAS PubMed PubMed Central Google Scholar - Kooijman S, Wang Y, Parlevliet ET et al (2015) Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 58(11):2637–2646. https://doi.org/10.1007/s00125-015-3727-0
Article CAS PubMed PubMed Central Google Scholar - Yan J, Yao B, Kuang H et al (2018) Liraglutide, sitagliptin and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and NAFLD. Hepatology (Baltimore, Md). https://doi.org/10.1002/hep.30320
Article CAS PubMed Google Scholar - Petit JM, Cercueil JP, Loffroy R et al (2017) Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the Lira-NAFLD Study. J Clin Endocrinol Metab 102(2):407–415. https://doi.org/10.1210/jc.2016-2775
Article PubMed Google Scholar - Armstrong MJ, Gaunt P, Aithal GP et al (2016) Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387(10019):679–690. https://doi.org/10.1016/s0140-6736(15)00803-x
Article CAS PubMed Google Scholar - Cuthbertson DJ, Irwin A, Gardner CJ et al (2012) Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon-like peptide-1 (GLP-1) receptor agonists. PLoS One 7(12):e50117. https://doi.org/10.1371/journal.pone.0050117
Article CAS PubMed PubMed Central Google Scholar - Ishii S, Iizuka K, Miller BC, Uyeda K (2004) Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci U S A 101(44):15597–15602. https://doi.org/10.1073/pnas.0405238101
Article CAS PubMed PubMed Central Google Scholar - Rijzewijk LJ, van der Meer RW, Smit JW et al (2008) Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52(22):1793–1799. https://doi.org/10.1016/j.jacc.2008.07.062
Article PubMed Google Scholar - Hammer S, Snel M, Lamb HJ et al (2008) Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol 52(12):1006–1012. https://doi.org/10.1016/j.jacc.2008.04.068
Article CAS PubMed Google Scholar - Wu L, Wang K, Wang W et al (2018) Glucagon-like peptide-1 ameliorates cardiac lipotoxicity in diabetic cardiomyopathy via the PPARalpha pathway. Aging Cell:e12763. https://doi.org/10.1111/acel.12763
Article PubMed PubMed Central Google Scholar - Leonardini A, D’Oria R, Incalza MA et al (2017) GLP-1 Receptor Activation Inhibits Palmitate-Induced Apoptosis via Ceramide in Human Cardiac Progenitor Cells. J Clin Endocrinol Metab 102(11):4136–4147. https://doi.org/10.1210/jc.2017-00970
Article PubMed Google Scholar - Levelt E, Pavlides M, Banerjee R et al (2016) Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol 68(1):53–63. https://doi.org/10.1016/j.jacc.2016.03.597
Article PubMed PubMed Central Google Scholar - Mahabadi AA, Berg MH, Lehmann N et al (2013) Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 61(13):1388–1395. https://doi.org/10.1016/j.jacc.2012.11.062
Article PubMed Google Scholar - van Eyk HJ, van Schinkel LD, Kantae V et al (2018) Caloric restriction lowers endocannabinoid tonus and improves cardiac function in type 2 diabetes. Nutr Diabetes 8(1):6–10. https://doi.org/10.1038/s41387-017-0016-7
Article CAS PubMed PubMed Central Google Scholar - Chau YY, Bandiera R, Serrels A et al (2014) Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol 16(4):367–375. https://doi.org/10.1038/ncb2922
Article CAS PubMed PubMed Central Google Scholar - Marso SP, Daniels GH, Brown-Frandsen K et al (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375(4):311–322. https://doi.org/10.1056/NEJMoa1603827
Article CAS PubMed PubMed Central Google Scholar - Goodman SN, Berlin JA (1994) The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med 121(3):200–206
Article CAS PubMed Google Scholar - Davies MJ, Bergenstal R, Bode B et al (2015) Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Jama 314(7):687–699. https://doi.org/10.1001/jama.2015.9676
Article CAS PubMed Google Scholar
Acknowledgements
We are grateful to all members of the MAGNA VICTORIA study group (see ESM) who assisted in recruitment of participants. We thank S. L. IJzermans (Department of Radiology, Leiden University Medical Center, the Netherlands) for assistance with image analysis of VAT and SAT, and B. Ladan-Eygenraam (Department of Internal Medicine, Leiden University Medical Center, the Netherlands) for assistance with processing of blood examinations. Some of the data were presented at American Diabetes Association Scientific Sessions in June 2017, Abstract no. 242.
Funding
Novo Nordisk A/S (Bagsvaerd, Denmark) funded this investigator-initiated study. The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
- Department of Radiology, Leiden University Medical Center, LUMC postzone C2S, Albinusdreef 2, 2333 ZA, Leiden, the Netherlands
Maurice B. Bizino, Paul de Heer, Ilona A. Dekkers, Elisabeth H. M. Paiman & Hildebrandus J. Lamb - Department of Medicine, Division of Endocrinology, Leiden University Medical Center, LUMC post zone C7Q, Albinusdreef 2, 2333 ZA, Leiden, the Netherlands
Ingrid M. Jazet, Huub J. van Eyk & Patrick C. N. Rensen - Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, Leiden, the Netherlands
Huub J. van Eyk & Patrick C. N. Rensen - Department of Medicine, Radboud University Medical Center, Nijmegen, the Netherlands
Johannes W. Smit
Authors
- Maurice B. Bizino
You can also search for this author inPubMed Google Scholar - Ingrid M. Jazet
You can also search for this author inPubMed Google Scholar - Paul de Heer
You can also search for this author inPubMed Google Scholar - Huub J. van Eyk
You can also search for this author inPubMed Google Scholar - Ilona A. Dekkers
You can also search for this author inPubMed Google Scholar - Patrick C. N. Rensen
You can also search for this author inPubMed Google Scholar - Elisabeth H. M. Paiman
You can also search for this author inPubMed Google Scholar - Hildebrandus J. Lamb
You can also search for this author inPubMed Google Scholar - Johannes W. Smit
You can also search for this author inPubMed Google Scholar
Contributions
All authors contributed to study concept and design and analysis and interpretation of data. EP, HE, MB and PH contributed to acquisition of data. IJ and JS supervised the MAGNA VICTORIA study, with HL as study director.. EP, HE, ID and MB drafted the manuscript. All authors contributed to critical revision of the manuscript and approved the final version of the manuscript to be published. MB and HL are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Correspondence toMaurice B. Bizino.
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bizino, M.B., Jazet, I.M., de Heer, P. et al. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation.Diabetologia 63, 65–74 (2020). https://doi.org/10.1007/s00125-019-05021-6
- Received: 07 June 2019
- Accepted: 28 August 2019
- Published: 05 November 2019
- Issue Date: January 2020
- DOI: https://doi.org/10.1007/s00125-019-05021-6