Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: a study of hospitalized children with acute respiratory tract infection in Guangzhou, China (original) (raw)
Introduction
Respiratory viral infections in humans, which can vary from common colds to severe respiratory disease, represent a significant global health burden and a pressing public health challenge in developing countries and among socioeconomically disadvantaged children in particular. Human coronaviruses (HCoV) OC43, 229E, NL63, and HKU1 are associated with a wide range of upper respiratory tract infections (URTI) and, occasionally, lower respiratory tract infections (LRTI), including pneumonia and bronchiolitis [[1](#ref-CR1 "Vabret A, Mourez T, Gouarin S et al (2003) An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis 36:985–989. https://doi.org/10.1086/374222
"),[2](#ref-CR2 "Woo PC, Lau SK, Tsoi HW et al (2005) Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis 192:1898–1907.
https://doi.org/10.1086/497151
"),[3](#ref-CR3 "Pene F, Merlat A, Vabret A et al (2003) Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis 37:929–932.
https://doi.org/10.1086/377612
"),[4](/article/10.1007/s10096-017-3144-z#ref-CR4 "Greenberg SB (2016) Update on human rhinovirus and coronavirus infections. Semin Respir Crit Care Med 37:555–571.
https://doi.org/10.1055/s-0036-1584797
")\], particularly in children \[[5](/article/10.1007/s10096-017-3144-z#ref-CR5 "Kuypers J, Martin ET, Heugel J et al (2007) Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70–e76.
https://doi.org/10.1542/peds.2006-1406
")\]. Although HCoV is widespread globally \[[6](#ref-CR6 "Zhao Q, Li S, Xue F et al (2008) Structure of the main protease from a global infectious human coronavirus, HCoV-HKU1. J Virol 82:8647–8655.
https://doi.org/10.1128/JVI.00298-08
"),[7](#ref-CR7 "van der Hoek L, Pyrc K, Berkhout B (2006) Human coronavirus NL63, a new respiratory virus. FEMS Microbiol Rev 30:760–773.
https://doi.org/10.1111/j.1574-6976.2006.00032.x
"),[8](/article/10.1007/s10096-017-3144-z#ref-CR8 "Woo PC, Lau SK, Chu CM et al (2005) Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79:884–895.
https://doi.org/10.1128/JVI.79.2.884-895.2005
")\], the frequency of detection of its four major strains varies significantly both by geography and over time \[[9](#ref-CR9 "Yip CC, Lam CS, Luk HK et al (2016) A six-year descriptive epidemiological study of human coronavirus infections in hospitalized patients in Hong Kong. Virol Sin 31:41–48.
https://doi.org/10.1007/s12250-016-3714-8
"),[10](#ref-CR10 "Esper F, Weibel C, Ferguson D et al (2006) Coronavirus HKU1 infection in the United States. Emerg Infect Dis 12:775–779.
https://doi.org/10.3201/eid1205.051316
"),[11](#ref-CR11 "Matoba Y, Abiko C, Ikeda T et al (2015) Detection of the human coronavirus 229E, HKU1, NL63, and OC43 between 2010 and 2013 in Yamagata, Japan. Jpn J Infect Dis 68:138–141.
https://doi.org/10.7883/yoken.JJID.2014.266
"),[12](#ref-CR12 "Gerna G, Percivalle E, Sarasini A et al (2007) Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J Clin Virol 38:244–250.
https://doi.org/10.1016/j.jcv.2006.12.008
"),[13](/article/10.1007/s10096-017-3144-z#ref-CR13 "Lee J, Storch GA (2014) Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J 33:814–820.
https://doi.org/10.1097/INF.0000000000000292
")\]. Despite these features of its epidemiology, few long-term studies of the prevalence of HCoV strains and their clinical manifestations have been undertaken \[[14](#ref-CR14 "Lau SK, Woo PC, Yip CC et al (2006) Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 44:2063–2071.
https://doi.org/10.1128/JCM.02614-05
"),[15](#ref-CR15 "Gerna G, Campanini G, Rovida F et al (2006) Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J Med Virol 78:938–949.
https://doi.org/10.1002/jmv.20645
"),[16](/article/10.1007/s10096-017-3144-z#ref-CR16 "Jevšnik M, Uršič T, Žigon N et al (2012) Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMC Infect Dis 12:365.
https://doi.org/10.1186/1471-2334-12-365
")\]. This paucity of evidence has led to an incomplete characterization of the epidemiology and clinical presentation of HCoV across different contexts.To expand the existing evidence base and provide new insights into the epidemiology and clinical manifestations of HCoV in a subtropical region, we performed a 7-year study of four HCoV strains among hospitalized pediatric patients with acute respiratory tract infection (ARTI) in Guangzhou, China.
Materials and methods
Sample collection
Throat swabs were collected from pediatric patients (≤ 14 years old) hospitalized with ARTI at The First Affiliated Hospital of Guangzhou Medical University and Sun Yat-Sen Memorial Hospital from July 2009 to June 2016. Both hospitals, each with nearly 2000 beds, were located in urban areas in Guangzhou, the capital city of a province with a humid subtropical climate. A case of HCoV was defined when a patient presented at least two of the following symptoms: cough, pharyngeal discomfort, rhinobyon, rhinorrhea, sneeze, dyspnea, or diagnosed with pneumonia by chest radiography during the previous week. The respiratory samples were refrigerated at 2–8 °C in viral transport medium, transported on ice to the State Key Laboratory of Respiratory Diseases, and analyzed immediately or stored at − 80 °C before analysis.
Cases were retrospectively categorized into three groups according to their clinical symptoms: URTI, influenza-like symptoms, and LRTI. Patients presenting with cough, expectoration, rhinorrhea, rhinobyon, sneeze, pharyngeal discomfort, or trachyphonia were classified as having URTI. Patients with fever (≥ 38 °C), chills, dizziness, headache, myalgia, or debilitation were classified as having influenza-like symptoms. Patients with bronchopneumonia, pneumonia, asthma, shortness of breath, chest tightness, chest pain, or abnormal pulmonary rales were classified as having LRTI. Bronchopneumonia and pneumonia were diagnosed with chest radiography. Other clinical symptoms were identified by common medical examinations and clinical descriptions.
Real-time PCR for HCoV detection
RNA was extracted from throat swab samples using the QIAamp Viral RNA Mini Kit (Qiagen, Shanghai, China), according to the manufacturer’s protocols. Samples were tested for four HCoV strains (HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1) using the TaqMan real-time PCR testing kit (Guangzhou HuYanSuo Medical Technology Co., Ltd.), as previously reported [[17](/article/10.1007/s10096-017-3144-z#ref-CR17 "Liu WK, Chen DH, Liu Q et al (2011) Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis 11:345. https://doi.org/10.1186/1471-2334-11-345
")\].Detection of common respiratory pathogens in HCoV-positive patients
HCoV-positive samples were simultaneously tested using TaqMan real-time PCR assays (Guangzhou HuYanSuo Medical Technology Co., Ltd.) for the following 11 respiratory pathogens: influenza A virus (Flu A), influenza B virus (Flu B), respiratory syncytial virus (RSV), human bocavirus (HBoV), adenovirus (ADV), human rhinovirus (HRV), human metapneumovirus (HMPV), enterovirus (EV), Mycoplasma pneumoniae (MP), Chlamydia pneumoniae (CP), and four types of human parainfluenza virus (HPIV).
Statistical analysis
All data were analyzed with SPSS statistical software (version 19.0; SPSS Inc., Chicago, IL), as described previously [[18](/article/10.1007/s10096-017-3144-z#ref-CR18 "Liu WK, Liu Q, Chen DH et al (2014) Epidemiology of acute respiratory infections in children in Guangzhou: a three-year study. PLoS One 9:e96674. https://doi.org/10.1371/journal.pone.0096674
")\]. The χ2 test and Fisher’s exact test were used for comparisons of data. All tests were two tailed and _p_ < 0.05 was considered statistically significant.Results
Detection of HCoV among patients with ARTI
A total of 11,399 hospitalized pediatric patients (≤ 14 years old) with ARTI were enrolled in this study between July 2009 and June 2016. The median age of the patients was 1.75 years (interquartile range, 0.75–3.83) and the male to female ratio was 1.82:1 (7361:4038). We found that 489 out of the 11,399 patients (4.3%) tested positive for HCoV. Of these, 346 (3%) were positive for HCoV-OC43, 65 (0.6%) for HCoV-229E, 60 (0.5%) for HCoV-NL63, and 38 (0.3%) for HCoV-HKU1. The median age of HCoV-positive patients was 1.25 years (interquartile range, 0.75–3) and the male to female ratio was 1.67:1 (306:183).
Co-infection in HCoV-positive patients
Samples from HCoV-positive patients were also tested for 11 other common respiratory pathogens. Of the 489 HCoV-positive patients, 258 (52.8%) were infected with only one HCoV strain, while 231 (47.2%) were found to be co-infected with one or more additional strains of HCoV or another respiratory pathogen (Table 1). Of these, the most frequently identified pathogens were Flu A (21.6%, 50/231) and RSV (21.6%, 50/231).
Table 1 Co-pathogen detection in human coronavirus-positive patients
Age distribution of HCoV-positive patients
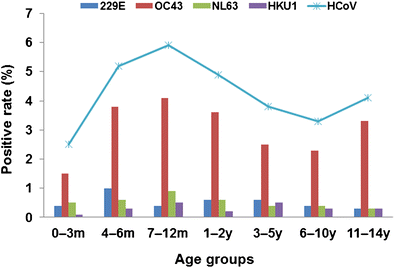
In this study, patients were divided into seven age groups: 0–3 months, 4–6 months, 7–12 months, 1–2 years, 3–5 years, 6–10 years, and 11–14 years. There were statistically significant differences in the prevalence of overall HCoV and of HCoV-OC43 by age group (p < 0.001). Patients aged 7–12 months had the highest prevalence of both overall HCoV (5.9%, 71/1203) and HCoV-OC43 (4.1%, 49/1203) compared with the other age groups (Fig. 1). There were no significant differences in the prevalence of HCoV-229E (p = 0.429) or HCoV-NL63 (p = 0.437). Too few cases of HCoV-HKU1 were identified to assess the age distribution for this strain.
Fig. 1
Age distribution of patients with human coronaviruses OC43, 229E, NL63, and HKU1. m: month(s); y: year(s)
Seasonal distribution of HCoV cases
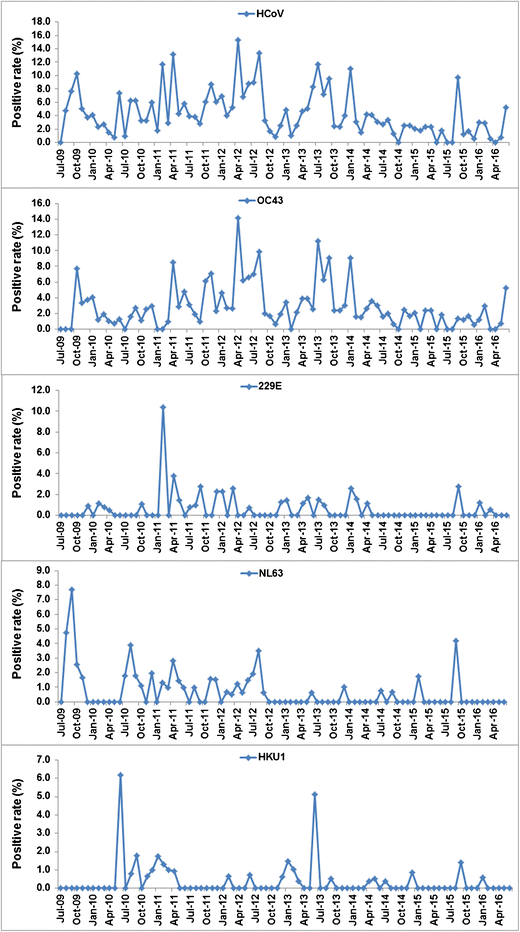
There was a clear seasonal pattern in the presentation of HCoV cases over the 7-year period (Fig. 2). The overall prevalence of HCoV among attending patients tended to be highest in the spring and autumn. During the study period, the months with the highest recorded prevalences were February 2011 (11.7%, 9/77), April 2011 (13.2%, 14/106), April 2012 (15.3%, 25/163), August 2012 (13.4%, 19/142), July 2013 (11.7%, 23/196), and January 2014 (11.0%, 17/154). These seasonal trends were primarily driven by cases of HCoV-OC43. The other strains had different seasonal patterns, with smaller, more sporadic outbreaks.
Fig. 2
Seasonal distribution of the four human coronavirus strains in pediatric patients with acute respiratory tract infection from July 2009 to June 2016. HCoV: human coronavirus; OC43: human coronavirus OC43; 229E: human coronavirus 229E; NL63: human coronavirus NL63; HKU1: human coronavirus HKU1
Clinical presentations of HCoV-positive patients
Table 2 shows the prevalence of clinical symptoms among HCoV-positive patients (n = 489) according to whether they had a single HCoV infection (n = 258) or were co-infected (n = 231), and according to the strain of HCoV detected.
Table 2 Clinical characteristics of human coronavirus-positive patients
There were statistically significant differences in the prevalence of pulmonary rales and fever according to whether a patient was co-infected. While the prevalence of pulmonary rales was higher among patients with a single HCoV infection (63.6%, 164/258) than among co-infected patients (54.5%, 126/231) (p = 0.043), fever was more prevalent among co-infected patients (66.2%, 153/231) than those with only one HCoV strain (55.4%, 143/258) (p = 0.014).
There were also significant differences in the prevalence of cough (p = 0.032), pneumonia (p = 0.026), and abnormal pulmonary rales (p = 0.002) according to the strain of HCoV detected.
Discussion
This retrospective study analyzed data from 11,399 hospitalized children (≤ 14 years old) presenting with ARTI in two large municipal hospitals over a 7-year period in Guangzhou, China. Given the present study’s duration and large sample size, our results represent an important addition to the evidence base on the epidemiology and clinical manifestations of HCoV. Of the 11,399 patients tested, we found that 489 (4.3%) were HCoV-positive and that the most prevalent strain of HCoV was OC43 (3.0%), followed by 229E (0.6%), NL63 (0.5%), and HKU1 (0.3%). These findings are consistent with the results of other studies around the world [[11](/article/10.1007/s10096-017-3144-z#ref-CR11 "Matoba Y, Abiko C, Ikeda T et al (2015) Detection of the human coronavirus 229E, HKU1, NL63, and OC43 between 2010 and 2013 in Yamagata, Japan. Jpn J Infect Dis 68:138–141. https://doi.org/10.7883/yoken.JJID.2014.266
"), [13](/article/10.1007/s10096-017-3144-z#ref-CR13 "Lee J, Storch GA (2014) Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J 33:814–820.
https://doi.org/10.1097/INF.0000000000000292
"), [19](/article/10.1007/s10096-017-3144-z#ref-CR19 "Dominguez SR, Robinson CC, Holmes KV (2009) Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol 81:1597–1604.
https://doi.org/10.1002/jmv.21541
")\]. The most common co-infecting pathogens among HCoV-positive patients were Flu A and RSV (Table [1](/article/10.1007/s10096-017-3144-z#Tab1)). Other recent studies have also reported that RSV, Flu A, and rhinoviruses are the most common pathogens that co-occur with HCoV, and that co-infection may influence the clinical presentation of HCoV-positive patients \[[4](/article/10.1007/s10096-017-3144-z#ref-CR4 "Greenberg SB (2016) Update on human rhinovirus and coronavirus infections. Semin Respir Crit Care Med 37:555–571.
https://doi.org/10.1055/s-0036-1584797
"), [5](/article/10.1007/s10096-017-3144-z#ref-CR5 "Kuypers J, Martin ET, Heugel J et al (2007) Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70–e76.
https://doi.org/10.1542/peds.2006-1406
"), [19](#ref-CR19 "Dominguez SR, Robinson CC, Holmes KV (2009) Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol 81:1597–1604.
https://doi.org/10.1002/jmv.21541
"),[20](#ref-CR20 "Gaunt ER, Hardie A, Claas EC et al (2010) Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947.
https://doi.org/10.1128/JCM.00636-10
"),[21](/article/10.1007/s10096-017-3144-z#ref-CR21 "Lu R, Yu X, Wang W et al (2012) Characterization of human coronavirus etiology in Chinese adults with acute upper respiratory tract infection by real-time RT-PCR assays. PLoS One 7:e38638.
https://doi.org/10.1371/journal.pone.0038638
")\].Consistent with studies conducted in other contexts, including America and Slovenia [[16](/article/10.1007/s10096-017-3144-z#ref-CR16 "Jevšnik M, Uršič T, Žigon N et al (2012) Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMC Infect Dis 12:365. https://doi.org/10.1186/1471-2334-12-365
"), [20](/article/10.1007/s10096-017-3144-z#ref-CR20 "Gaunt ER, Hardie A, Claas EC et al (2010) Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947.
https://doi.org/10.1128/JCM.00636-10
")\], our results showed that the prevalence of HCoV was highest among patients aged 7–12 months (Fig. [1](/article/10.1007/s10096-017-3144-z#Fig1)). This increased vulnerability to respiratory pathogens may be attributable to increased contact with pathogens as infants begin to explore their environment or the waning of maternal antibody levels in infants while the immune system remains underdeveloped \[[22](#ref-CR22 "Huijskens EG, Biesmans RC, Buiting AG et al (2012) Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol J 9:276.
https://doi.org/10.1186/1743-422X-9-276
"),[23](#ref-CR23 "van der Zalm MM, van Ewijk BE, Wilbrink B et al (2009) Respiratory pathogens in children with and without respiratory symptoms. J Pediatr 154:396–400.e1.
https://doi.org/10.1016/j.jpeds.2008.08.036
"),[24](/article/10.1007/s10096-017-3144-z#ref-CR24 "Suryadevara M, Cummings E, Bonville CA et al (2011) Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clin Pediatr (Phila) 50:513–517.
https://doi.org/10.1177/0009922810394834
")\].HCoV is widespread globally and patterns of outbreaks vary according to locations and seasonal factors [[4](/article/10.1007/s10096-017-3144-z#ref-CR4 "Greenberg SB (2016) Update on human rhinovirus and coronavirus infections. Semin Respir Crit Care Med 37:555–571. https://doi.org/10.1055/s-0036-1584797
")\]. Our study found that HCoV prevalence among patients presenting with ARTI in Guangzhou over a 7-year period was highest in the spring and autumn (Fig. [2](/article/10.1007/s10096-017-3144-z#Fig2)). This stands in contrast to other studies which find higher prevalence of HCoV infection in winter and spring \[[16](/article/10.1007/s10096-017-3144-z#ref-CR16 "Jevšnik M, Uršič T, Žigon N et al (2012) Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMC Infect Dis 12:365.
https://doi.org/10.1186/1471-2334-12-365
"), [20](/article/10.1007/s10096-017-3144-z#ref-CR20 "Gaunt ER, Hardie A, Claas EC et al (2010) Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947.
https://doi.org/10.1128/JCM.00636-10
")\]. We also found different seasonal prevalence patterns for each of the four HCoV strains, with peak frequencies of 229E, NL63, and OC43 occurring mostly in the spring and autumn in Guangzhou, although OC43 had lower peaks appearing in July 2012 and 2013, however (Fig. [2](/article/10.1007/s10096-017-3144-z#Fig2)). Other studies conducted in Hong Kong have shown that, while the highest frequencies of NL63 and OC43 cases occurred in autumn and winter during the period 2005–2007 \[[25](/article/10.1007/s10096-017-3144-z#ref-CR25 "Leung TF, Li CY, Lam WY et al (2009) Epidemiology and clinical presentations of human coronavirus NL63 infections in Hong Kong children. J Clin Microbiol 47:3486–3492.
https://doi.org/10.1128/JCM.00832-09
")\], OC43 and HKU1 cases peaked in winter and NL63 prevalence was highest in summer and autumn during the period 2004–2005 \[[14](/article/10.1007/s10096-017-3144-z#ref-CR14 "Lau SK, Woo PC, Yip CC et al (2006) Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 44:2063–2071.
https://doi.org/10.1128/JCM.02614-05
")\]. In the United States, 229E, OC43, and HKU1 have been shown to follow different seasonal patterns, with outbreaks of 229E occurring in winter, OC43 in spring and autumn, and HKU1 in summer \[[20](/article/10.1007/s10096-017-3144-z#ref-CR20 "Gaunt ER, Hardie A, Claas EC et al (2010) Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947.
https://doi.org/10.1128/JCM.00636-10
")\]. Although these seasonal patterns vary between countries and over time, it is apparent across all studies that the prevalence of HCoV among children is lowest in early summer.Patients with HCoV infections presented a wide spectrum of respiratory symptoms. When we compared the clinical presentations of patients with a single HCoV infection to those with co-infections, we found that abnormal pulmonary rales occurred more frequently in the former group, while fever was more prevalent in the latter (Table 2). The results, therefore, indicate that patients infected with more than one respiratory pathogen are more likely to develop fever. Furthermore, abnormal pulmonary rales were more frequently detected among patients infected with OC43 than those infected with other strains. This suggests that HCoV-OC43 is more closely associated with LRTI. Patients with HCoV-OC43 also had the highest prevalence of broncho-pneumonia and asthma, although this was not significantly higher than among patients with other strains (Table 2). Our results are consistent with the findings of Lee and Storch [[13](/article/10.1007/s10096-017-3144-z#ref-CR13 "Lee J, Storch GA (2014) Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J 33:814–820. https://doi.org/10.1097/INF.0000000000000292
")\] that HCoV-NL63 and HCoV-OC43 are associated with LRTI in children. However, Kuypers et al. \[[5](/article/10.1007/s10096-017-3144-z#ref-CR5 "Kuypers J, Martin ET, Heugel J et al (2007) Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70–e76.
https://doi.org/10.1542/peds.2006-1406
")\] have found that, although HCoV-OC43 may be associated with asthma and some symptoms related to LRTI, other pathogens such as RSV may be more strongly implicated in cases of severe LRTI \[[26](/article/10.1007/s10096-017-3144-z#ref-CR26 "Kristoffersen AW, Nordbø SA, Rognlien AG et al (2011) Coronavirus causes lower respiratory tract infections less frequently than RSV in hospitalized Norwegian children. Pediatr Infect Dis J 30:279–283.
https://doi.org/10.1097/INF.0b013e3181fcb159
")\]. Recent studies have also shown that the most prevalent URTI symptoms among HCoV-positive individuals are fever, cough, sore throat, and headache \[[1](/article/10.1007/s10096-017-3144-z#ref-CR1 "Vabret A, Mourez T, Gouarin S et al (2003) An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis 36:985–989.
https://doi.org/10.1086/374222
"), [19](/article/10.1007/s10096-017-3144-z#ref-CR19 "Dominguez SR, Robinson CC, Holmes KV (2009) Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol 81:1597–1604.
https://doi.org/10.1002/jmv.21541
")\], and that LRTI including pneumonia and bronchiolitis also occasionally co-occur with HCoV \[[1](/article/10.1007/s10096-017-3144-z#ref-CR1 "Vabret A, Mourez T, Gouarin S et al (2003) An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis 36:985–989.
https://doi.org/10.1086/374222
"), [5](/article/10.1007/s10096-017-3144-z#ref-CR5 "Kuypers J, Martin ET, Heugel J et al (2007) Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70–e76.
https://doi.org/10.1542/peds.2006-1406
")\]. However, influenza-like symptoms were uncommon in our sample of HCoV-positive patients in this study.Our study has several strengths, including its large sample size and long duration. Furthermore, given that few studies to date have simultaneously tested for all four strains of HCoV in ARTI pediatric patients, the present study addresses an important gap in the literature.
This study had some limitations, however. First, selection bias may have occurred due to the lack of healthy subjects without ARTI. Second, collecting biological samples using oropharyngeal swabs may be less reliable for detecting the presence of HCoV and other pathogens than obtaining bronchoalveolar lavage fluid. One advantage of this method, however, was that it is non-invasive and more suitable for routine analysis.
In conclusion, the four strains of HCoV investigated in the present study are common among pediatric patients with ARTI in Guangzhou, China, and are often found alongside other respiratory pathogens. HCoV infection may cause a broad spectrum of symptoms, ranging from common cold-like symptoms, to influenza-like symptoms, asthma, and even pneumonia. The present study underscores the importance of HCoV infection in the etiology of pediatric ARTI, its relevance in clinical practice, and the pressing need to improve surveillance and detection in developing country contexts.
References
- Vabret A, Mourez T, Gouarin S et al (2003) An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin Infect Dis 36:985–989. https://doi.org/10.1086/374222
Article PubMed Google Scholar - Woo PC, Lau SK, Tsoi HW et al (2005) Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis 192:1898–1907. https://doi.org/10.1086/497151
Article CAS PubMed Google Scholar - Pene F, Merlat A, Vabret A et al (2003) Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis 37:929–932. https://doi.org/10.1086/377612
Article PubMed Google Scholar - Greenberg SB (2016) Update on human rhinovirus and coronavirus infections. Semin Respir Crit Care Med 37:555–571. https://doi.org/10.1055/s-0036-1584797
Article PubMed Google Scholar - Kuypers J, Martin ET, Heugel J et al (2007) Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics 119:e70–e76. https://doi.org/10.1542/peds.2006-1406
Article PubMed Google Scholar - Zhao Q, Li S, Xue F et al (2008) Structure of the main protease from a global infectious human coronavirus, HCoV-HKU1. J Virol 82:8647–8655. https://doi.org/10.1128/JVI.00298-08
Article CAS PubMed PubMed Central Google Scholar - van der Hoek L, Pyrc K, Berkhout B (2006) Human coronavirus NL63, a new respiratory virus. FEMS Microbiol Rev 30:760–773. https://doi.org/10.1111/j.1574-6976.2006.00032.x
Article PubMed Google Scholar - Woo PC, Lau SK, Chu CM et al (2005) Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79:884–895. https://doi.org/10.1128/JVI.79.2.884-895.2005
Article CAS PubMed PubMed Central Google Scholar - Yip CC, Lam CS, Luk HK et al (2016) A six-year descriptive epidemiological study of human coronavirus infections in hospitalized patients in Hong Kong. Virol Sin 31:41–48. https://doi.org/10.1007/s12250-016-3714-8
Article PubMed Google Scholar - Esper F, Weibel C, Ferguson D et al (2006) Coronavirus HKU1 infection in the United States. Emerg Infect Dis 12:775–779. https://doi.org/10.3201/eid1205.051316
Article PubMed PubMed Central Google Scholar - Matoba Y, Abiko C, Ikeda T et al (2015) Detection of the human coronavirus 229E, HKU1, NL63, and OC43 between 2010 and 2013 in Yamagata, Japan. Jpn J Infect Dis 68:138–141. https://doi.org/10.7883/yoken.JJID.2014.266
Article PubMed Google Scholar - Gerna G, Percivalle E, Sarasini A et al (2007) Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J Clin Virol 38:244–250. https://doi.org/10.1016/j.jcv.2006.12.008
Article CAS PubMed Google Scholar - Lee J, Storch GA (2014) Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J 33:814–820. https://doi.org/10.1097/INF.0000000000000292
Article PubMed Google Scholar - Lau SK, Woo PC, Yip CC et al (2006) Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 44:2063–2071. https://doi.org/10.1128/JCM.02614-05
Article CAS PubMed PubMed Central Google Scholar - Gerna G, Campanini G, Rovida F et al (2006) Genetic variability of human coronavirus OC43-, 229E-, and NL63-like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J Med Virol 78:938–949. https://doi.org/10.1002/jmv.20645
Article CAS PubMed Google Scholar - Jevšnik M, Uršič T, Žigon N et al (2012) Coronavirus infections in hospitalized pediatric patients with acute respiratory tract disease. BMC Infect Dis 12:365. https://doi.org/10.1186/1471-2334-12-365
Article PubMed PubMed Central Google Scholar - Liu WK, Chen DH, Liu Q et al (2011) Detection of human bocavirus from children and adults with acute respiratory tract illness in Guangzhou, southern China. BMC Infect Dis 11:345. https://doi.org/10.1186/1471-2334-11-345
Article CAS PubMed PubMed Central Google Scholar - Liu WK, Liu Q, Chen DH et al (2014) Epidemiology of acute respiratory infections in children in Guangzhou: a three-year study. PLoS One 9:e96674. https://doi.org/10.1371/journal.pone.0096674
Article PubMed PubMed Central Google Scholar - Dominguez SR, Robinson CC, Holmes KV (2009) Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol 81:1597–1604. https://doi.org/10.1002/jmv.21541
Article PubMed PubMed Central Google Scholar - Gaunt ER, Hardie A, Claas EC et al (2010) Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947. https://doi.org/10.1128/JCM.00636-10
Article CAS PubMed PubMed Central Google Scholar - Lu R, Yu X, Wang W et al (2012) Characterization of human coronavirus etiology in Chinese adults with acute upper respiratory tract infection by real-time RT-PCR assays. PLoS One 7:e38638. https://doi.org/10.1371/journal.pone.0038638
Article CAS PubMed PubMed Central Google Scholar - Huijskens EG, Biesmans RC, Buiting AG et al (2012) Diagnostic value of respiratory virus detection in symptomatic children using real-time PCR. Virol J 9:276. https://doi.org/10.1186/1743-422X-9-276
Article CAS PubMed PubMed Central Google Scholar - van der Zalm MM, van Ewijk BE, Wilbrink B et al (2009) Respiratory pathogens in children with and without respiratory symptoms. J Pediatr 154:396–400.e1. https://doi.org/10.1016/j.jpeds.2008.08.036
Article PubMed Google Scholar - Suryadevara M, Cummings E, Bonville CA et al (2011) Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clin Pediatr (Phila) 50:513–517. https://doi.org/10.1177/0009922810394834
Article Google Scholar - Leung TF, Li CY, Lam WY et al (2009) Epidemiology and clinical presentations of human coronavirus NL63 infections in Hong Kong children. J Clin Microbiol 47:3486–3492. https://doi.org/10.1128/JCM.00832-09
Article CAS PubMed PubMed Central Google Scholar - Kristoffersen AW, Nordbø SA, Rognlien AG et al (2011) Coronavirus causes lower respiratory tract infections less frequently than RSV in hospitalized Norwegian children. Pediatr Infect Dis J 30:279–283. https://doi.org/10.1097/INF.0b013e3181fcb159
Article PubMed Google Scholar