Administration of a cAMP Phosphodiesterase 4 Inhibitor Enhances Antidepressant-Induction of BDNF mRNA in Rat Hippocampus (original) (raw)
Main
Although numerous preclinical studies have been undertaken to identify a common action of antidepressant drugs, the mechanism(s) which underlies the therapeutic action of these treatments remains unknown. One likely target of antidepressant administration is the cAMP signal transduction system, because chronic, but not acute, antidepressant treatment up-regulates this intracellular cascade at several levels (Nestler et al. 1989; Ozawa and Rasenick 1991; Nibuya et al. 1996; Duman et al. 1997). On the other hand, the therapeutic response to antidepressants requires several weeks of treatment, suggesting that long-term neuronal adaptations mediated by changes in gene expression may be important to the therapeutic action of antidepressant treatment (Heninger and Charney 1987; Duman et al. 1995, 1997). Based on these findings, it is postulated that neural plasticity induced by activation of the cAMP signal transduction cascade may be involved in the therapeutic action of antidepressant drugs.
Brain-derived neurotrophic factor (BDNF) is reported to play an important role in the survival of mature neurons as well as damaged neurons in the central nervous system (Ghosh et al. 1994; Mamonous et al. 1994). Recent studies revealed that the activation of the cAMP system, as well as β-adrenergic receptors that couple to this system, is closely involved in the regulation of BDNF mRNA expression (Zafra et al. 1990, 1992; Condorelli et al. 1994; Nibuya et al. 1996; Bishop et al. 1997). We have reported that chronic administration (21 days) of several different types of antidepressant increases the expression of BDNF mRNA in rat frontal cortex and hippocampus (Nibuya et al. 1995, 1996). In addition, local infusion of BDNF into midbrain has an antidepressant like effect in behavioral models of depression (Siuciak et al. 1997). Conversely, levels of BDNF mRNA are reported to be decreased by acute as well as chronic restraint stress in rat hippocampus (Smith et al. 1995). Taken together, these findings support the possibility that activation of the cAMP system and increased expression of BDNF contribute to the neural adaptations that underlie the action of antidepressant treatment.
This model suggests that agents which activate the β-adrenergic receptor and cAMP intracellular cascade would potentially be effective antidepressant agents. One approach is to use an inhibitor of phosphodiesterase (PDE), the enzymes responsible for the breakdown of cAMP. Although there are many different types of PDE, cAMP PDE type 4 may be one relevant target (Beavo et al. 1994; Conti et al. 1995; Conti and Jin in press). This is based on reports that PDE4 inhibitors, such as rolipram, have antidepressant effects in behavioral models of depression (Wachtel and Schneider 1986; Griebel et al. 1991; O'Donnell 1993) and clinical trials demonstrating that rolipram has antidepressant efficacy in depressed patients (Bobon et al. 1988; Fleischhacker et al. 1992). In spite of these reports, the use of rolipram for the treatment of depression has been limited by its side effects.
The present study was undertaken to examine the influence of acute or chronic coadministration of two different PDE4 inhibitors with an antidepressant (desipramine or Org 4428) on the expression of BDNF mRNA. Desipramine as well as Org 4428 is a reuptake inhibitor with higher affinity for norepinephrine transporter. This work extends a preliminary report on the influence of rolipram on the expression of BDNF mRNA in rat hippocampus (Nibuya et al. 1996).
MATERIALS AND METHODS
Animals and treatment paradigms. Male Sprague-Dawley rats (200 g; Japan Charles River, Japan) were group housed and maintained on a 12-h light cycle with food and water freely available. For the studies of coadministration of a PDE4 inhibitor and an antidepressant, groups of rats were administered desipramine (15 mg/kg), Org 4428 (20 mg/kg), rolipram (1.25 mg/kg), Ro 20–1724 (12.5 mg/kg), or vehicle (1% Tween 80) once daily for 1, 7, 14, or 21 d via intraperitoneal injection and sacrificed by decapitation 2 h after the last treatment. All animals use procedures were approved by the Yamagata University Animal Care Committee.
The brains were removed and immediately frozen for in situ hybridization, or sections of hippocampus were dissected for RNA extraction and Northern blot analysis as described below. The same animals were used for in situ and Northern blot experiments. The drugs used for these studies were obtained from the following sources: rolipram was a generous gift of Solvay-Meiji Pharmaceutical Company (Tokyo, Japan), Ro 20–1724 was a generous gift of Hoffmann-LaRoche INC (Nutley, NJ), desipramine was purchased from Sigma, and Org 4428 was a generous gift of Organon Pharmaceutical Company (Oss, The Netherlands).
Northern Blot Analysis
Total RNA was isolated from sections of hippocampus by the guanidine isothiocyanate/cesium chloride centrifugation method, as previously described (Morinobu et al. 1995; Nibuya et al. 1995). The rat BDNF cDNA clone was provided by Regeneron (Tarrytown, NY). The BDNF cDNA, subcloned into pBluescript SK- (Stratagene, La Jolla, CA), was linearlized with Bpu1102 I (Takara Shuzo CO., LTD, Otsu, Japan) and uniformly radiolabeled riboprobes corresponding to the antisense strand (bp 507–833) were generated with T7 RNA polymerase and 32P-UTP (Dupont NEN, Boston, MA) as previously described (Nibuya et al. 1995). Briefly, 20 μg of total RNA were electrophoresed on a 1% agarose gel and the RNA was transferred to nitrocellulose filters. The resulting filters were then incubated with the 32P-labeled BDNF riboprobes for 18 h at 65°C and washed twice in 2 × SSC (SSC: 0.15 M NaCl, 0.015 M sodium citrate at pH 7.0), 0.1% sodium dodecyl sulfate (SDS) at 65°C for 15 min, and then in 0.2 × SSC, 0.1 % SDS at 65°C for 15 min. Levels of total RNA for each lane were determined by reprobing the nitrocellulose filters with a 32P-labeled cyclophilin cDNA probe that was radiolabeled using a random prime kit (Amersham, Beckinghamshire, England) as previously described (Morinobu et al. 1995). The levels of BDNF mRNA were divided by cyclophilin mRNA to account for differences in the amount of RNA per lane. The results are expressed as percent of sham.
In Situ Hybridization Analysis
Analysis of BDNF mRNA by in situ hybridization was conducted as previously described (Nibuya et al. 1995, 1996). Briefly, coronal sections of frozen brain (18 μm) were cut on a cryostat and stored at −70°C. Tissue sections were thaw mounted on RNase-free Probon slides (Fisher Biotech, Pittsburgh, PA), fixed, and dried. The 35S-radiolabeled BDNF riboprobes were hybridized with the brain sections (2 × 106 CPM per section) for 18 h at 55°C in hybridization buffer (50% formamide, 0.6 M NaCl, 10 mM Tris, 1x Denhardt's solution, 2 mM EDTA, 10 mM DTT, 10% dextran sulfate, 200 μg/ml salmon sperm DNA, and 250 μg/ml tRNA). The sections were washed in 2 × SSC at room temperature, and then treated with 20 μg/ml RNase A for 30 min in 0.5 M NaCl, 10 mM Tris, and 1 mM EDTA at room temperature. The sections were then washed twice in 0.2 × SSC at 55°C, 30 min per wash. The sections were dried, exposed to Hyperfilm (Amersham, Arlington Heights, IL).
Data Analysis
Levels of BDNF and cyclophilin mRNA were determined by outlining the band on Northern blots or the regions of interest in situ hybridization sections and then quantified on a Macintosh-based ATTO Image analysis program, Version 3.01 (Tokyo, Japan). The results were then subjected to statistical analysis. Experiments containing groups of three or more were subjected to ANOVA, with a significance level of p < .05, and Fisher's post hoc test. Experiments containing groups of two rats were subjected to the Student's _t_-test, with a significance level of p < .05.
RESULTS
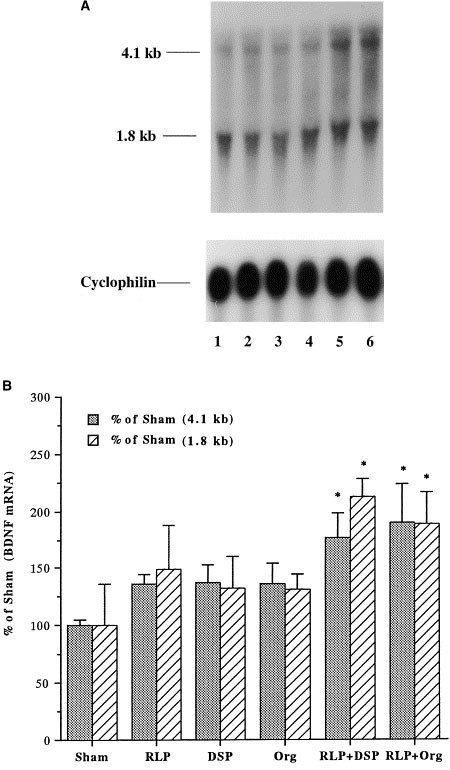
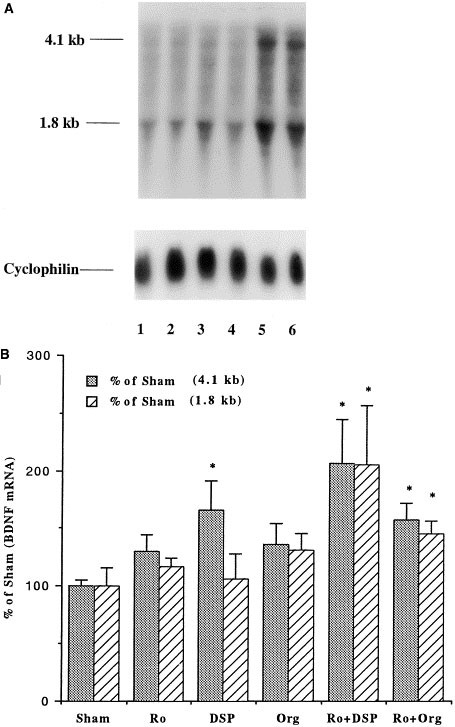
The influence of chronic coadministration (21 days) of a PDE4 inhibitor (rolipram or Ro 20–1724) and an antidepressant (desipramine or Org 4428) on BDNF mRNA was examined by Northern blot analysis. These studies demonstrate that all four different combinations tested, including rolipram + desipramine, rolipram + Org 4428, Ro 20–1724 + desipramine, and Ro 20–1724 + Org 4428, significantly increased the expression of BDNF mRNA in hippocampus relative to chronic administration of vehicle (Figures 1 and 2). Levels of both major forms of BDNF mRNA (4.1 and 1.8 kb) by the coadministration paradigm were significantly higher than those by the vehicle treatment. In contrast, statistical analyses indicated that BDNF induction by all four different combinations for 21 d was not greater than that by the two different single antidepressant treatment (Figures 1 and 2). Two BDNF mRNA bands, 4.1 kb and 1.8 kb, result from alternative polyadenylation sites within the 3′ untranslated region (Timmusk et al. 1993). In addition, chronic administration of either a PDE4 inhibitor or an antidepressant alone did not significantly increase the expression of BDNF mRNA in the hippocampus, although there was a tendency for an upregulation by the individual treatments (Figures 1 and 2).
Figure 1
Influence of chronic coadministration (21 days) of rolipram (RLP) with desipramine (DSP) or Org 4428 (Org) on BDNF mRNA in rat hippocampus. Levels of BDNF were measured 2 h after the last injection by Northern blot analysis. (A) Representative Northern blots for chronic coadministration: 1, sham (vehicle); 2, RLP; 3, DSP; 4, Org; 5, RLP + DSP; and 6, RLP + Org. (B) Quantitation of BDNF mRNA. Levels of mRNA were quantified by densitometry and standardized for the amount of mRNA in each lane by reprobing the filters with radiolabeled cyclophilin. Data are presented as percentage of sham (vehicle treatment) and are the mean ± S.E.M. of 5 to 6 rats for each treatment group. *p < .05 compared with vehicle control (ANOVA and Fisher's test).
Figure 2
Influence of chronic coadministration (21 days) of Ro 20–1724 (Ro) with desipramine (DSP) or Org 4428 (Org) on BDNF mRNA in rat hippocampus. Levels of BDNF were measured 2 h after the last injection by Northern blot analysis. (A) Representative Northern blots for chronic coadministration: 1, sham (vehicle); 2, Ro; 3, DSP; 4, Org; 5, Ro + DSP; and 6, Ro + Org. (B) Quantitation of BDNF mRNA. Levels of mRNA were quantified by densitometry and standardized for the amount of mRNA in each lane by reprobing the filters with radiolabeled cyclophilin. Data are presented as percentage of sham (vehicle treatment) and are the mean ± S.E.M. of 5 to 6 rats for each treatment group. *p < .05 compared with vehicle control (ANOVA and Fisher's test).
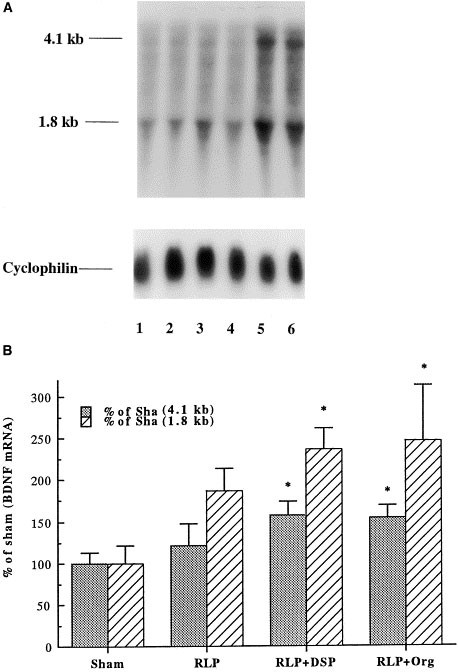
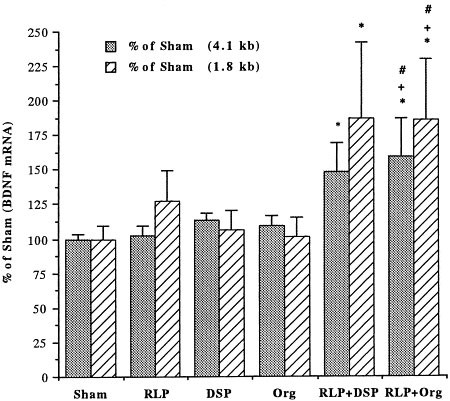
The time course for upregulation of BDNF mRNA by the coadministration paradigm was examined. We first examined the influence of acute treatments. The PDE4 inhibitor and antidepressants were administered and two hours later levels of BDNF mRNA were determined. Acute coadministration of a PDE inhibitor and an antidepressant did not significantly influence levels of BDNF mRNA in hippocampus (Table 1). This included the four different combinations tested in the chronic study. In addition, acute administration of either a PDE4 inhibitor or an antidepressant alone did not significantly influence levels of BDNF mRNA (Table 1). We next determined the influence of the coadministration paradigm for longer treatment times. Statistical analyses indicated that the BDNF induction (both 4.1 and 1.8 kb transcript) by coadministration of rolipram with either desipramine or Org 4428 for either 7 or 14 d was significantly greater than that by the vehicle treatment (Figures 3 and 4). In addition, the BDNF induction by coadministration of rolipram with Org 4428 for 14 d was significantly higher than that by desipramine or Org 4428 (Figure 4). In contrast, administration of rolipram alone for 7 or 14 d or either antidepressant alone for 14 d did not significantly influence BDNF levels. Because antidepressant treatment alone for 14 d had no significant effect on BDNF mRNA, the influence of 7 d antidepressant treatment alone was not determined.
Table 1 Influence of Acute Treatment with a PDE4 Inhibitor and/or an Antidepressant on BDNF mRNA in Rat Hippocampus by Northern Blot Analysis
Figure 3
Influence of coadministration of rolipram (RLP) with desipramine (DSP) or Org 4428 (Org) for 7 d on BDNF mRNA in rat hippocampus. Levels of BDNF were measured 2 h after the last injection by Northern blot analysis. (A) Representative Northern blots for chronic coadministration: 1, sham (vehicle); 2, RLP; 3, RLP + DSP; and 4, RLP + Org. (B) Quantitation of BDNF mRNA. Levels of mRNA were quantified by densitometry and standardized for the amount of mRNA in each lane by reprobing the filters with radiolabeled cyclophilin. Data are presented as percentage of sham (vehicle treatment) and are the mean ± S.E.M. of 4 to 5 rats for each treatment group. *p < .05 compared with vehicle control (ANOVA and Fisher's test).
Figure 4
Influence of coadministration of rolipram (RLP) with desipramine (DSP) or Org 4428 (Org) for 14 d on BDNF mRNA in rat hippocampus. Levels of BDNF were measured 2 h after the last injection by Northern blot analysis. Levels of mRNA were quantified by densitometry and standardized for the amount of mRNA in each lane by reprobing the filters with radiolabeled cyclophilin. Data are presented as percentage of sham (vehicle treatment) and are the mean ± S.E.M. of 5 to 7 rats for each treatment group. *p < .05 compared with vehicle control, +p < .05 compared with desipramine treatment, and #p < .05 compared with Org 4428 treatment (ANOVA and Fisher's test).
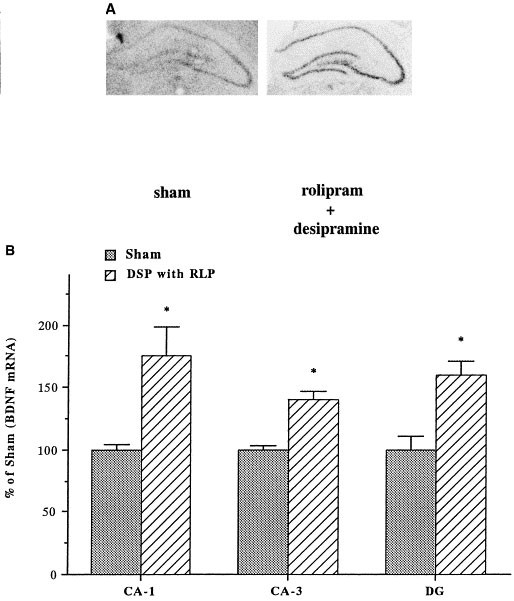
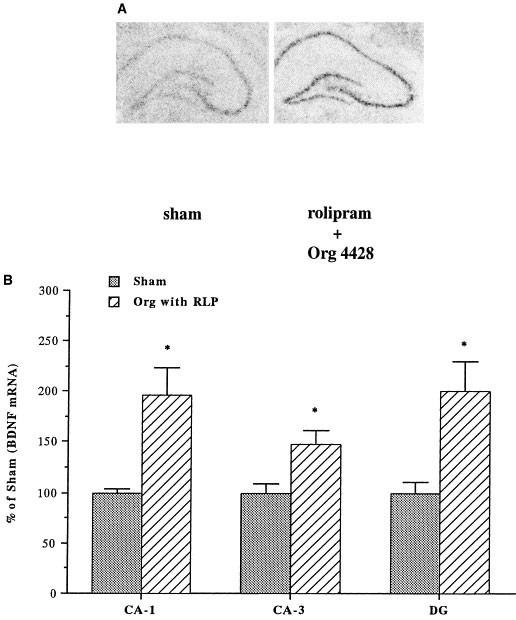
To elucidate the hippocampal cell layers where the expression of BDNF is regulated, we used in situ hybridization. Because we observed a significant upregulation of BDNF by Northern blot analysis after coadministration of rolipram with either desipramine or Org 4428 for 7 days, this treatment paradigm was used. Coadministration of rolipram and either desipramine or Org 4428 significantly increased levels of BDNF mRNA in the major subfields of hippocampus, including the CA1 and CA3 pyramidal cell layers and the dentate gyrus granule cell layer (Figures 5 and 6). In contrast, acute coadministration of rolipram with either desipramine or Org 4428 did not significantly influence levels of BDNF mRNA in these subfields of hippocampus (data not shown).
Figure 5
Influence of coadministration of rolipram with desipramine for 7 d on BDNF mRNA in CA1 and CA3 pyramidal cell layers, and dentate gyrus (DG) granule cell layers. (A) Representative in situ hybridization autoradiogram for coadministration, left: sham (vehicle), right: rolipram + desipramine. (B) Quantitation of BDNF mRNA. Levels of BDNF mRNA were determined 2 h after the last injection by quantitative densitometry on in situ hybridization autoradiograms. Data are presented as percentage of sham (vehicle treatment) and are the mean ± S.E.M. of 4 to 5 rats for each treatment group. *p < .05 compared with vehicle control (the Student's _t_-test).
Figure 6
Influence of coadministration of rolipram with Org 4428 for 7 d on BDNF mRNA in CA1 and CA3 pyramidal cell layers, and dentate gyrus granule cell layers. (A) Representative in situ hybridization autoradiogram for coadministration, left: sham (vehicle), right: rolipram + Org 4428. (B) Levels of BDNF mRNA were determined 2 h after the last injection by quantitative densitometry on in situ hybridization autoradiograms. Data are presented as percentage of sham (vehicle treatment) and are the mean ± S.E.M. of 4 to 5 rats for each treatment group. *p < .05 compared with vehicle control (the Student's _t_-test).
DISCUSSION
This study demonstrates that chronic, but not acute, coadministration of a PDE4 inhibitor with an antidepressant significantly increases the expression of BDNF mRNA in rat hippocampus. The PDE4 inhibitors and antidepressant drugs tested influence the cAMP signal transduction system via different mechanisms: the PDE4 inhibitor blocks the breakdown of cAMP and the antidepressant increases norepinephrine levels, thereby activating the β-adrenergic receptors coupled to this second messenger pathway. In this way, repeated coadministration of a PDE4 inhibitor with an antidepressant could potentiate cAMP-mediated signal transduction and subsequently produce the marked induction of BDNF mRNA expression. This explanation is supported by previous studies demonstrating that activation of β-adrenergic receptors and the cAMP signal transduction cascade increase BDNF mRNA levels in primary cultures of brain and in cultured cell lines (Zafra et al. 1992; Condorelli et al. 1994; Bishop et al. 1997). On the other hand, it is also plausible that a metabolic interaction between rolipram and antidepressant drugs may increase plasma levels of the antidepressant and subsequently lead a greater upregulation of the BDNF mRNA expression. Although there has been no report examining metabolic interaction between these drugs, we can not rule out the possibility that the significant induction of BDNF mRNA observed in this study may reflect increased plasma levels of antidepressants.
Local infusion of BDNF into cerebral cortex is reported to prevent the damage of 5-HT axons induced by neurotoxin treatment and to promote the sprouting of these neurons (Mamonous et al. 1994). BDNF is also reported to potentiate 5-HT-mediated signal transduction in rat brain and 5-HT-related behaviors (Martin-Iverson et al. 1994; Siuciak et al. 1994). In addition, Smith and co-workers (1995), and Ueyama and colleagues (1997) have demonstrated that chronic stress down-regulates the expression of BDNF mRNA in rat hippocampus, raising the possibility that decreased expression of BDNF might contribute to the atrophy and degeneration of neurons in response to chronic stress (see Duman et al. 1997). However, Kuroda and McEwen (1998) failed to observe a similar effect in response to restraint stress, suggesting that down-regulation of BDNF may not be a common action of all types of stress. The results of this study suggest that coadministration of a PDE4 inhibitor could potentiate neurotrophic effects induced by antidepressant treatment and thereby facilitate antidepressant efficacy. However, further studies will be required to determine the exact neurotrophic effects of a PDE4 inhibitor when coadministered with an antidepressant.
In a previous study, we demonstrated that expression of BDNF mRNA is significantly increased by coadministration of rolipram with imipramine for 7 days, but not by either drug alone for the same time (Nibuya et al. 1996). The current study confirms and extends this work. While 10 mg/kg of rolipram was administered in the previous study, 1.25 mg/kg of rolipram was used in the present study. Preclinical studies undertaken to examine the antidepressant efficacy of rolipram demonstrate that a dose of 0.3 to 1.25 mg/kg of rolipram is sufficient to exert an antidepressant effect in rats (Przegalinski and Bigajska 1983; O'Donnell 1993). In contrast, the higher dose of rolipram (10 mg/kg) used in the previous study has some adverse effects, including decreased food intake and weight loss. In our previous report we also found that chronic administration of rolipram (2 mg/kg, 14 days) resulted in a significant upregulation of BDNF mRNA (Nibuya et al. 1996). However, in the present study, chronic administration of rolipram at a lower dose (1.25 mg/kg) for 14 or 21 d did not significantly influence levels of BDNF mRNA, although there was a tendency for increased expression. It is possible that the slightly higher dose of rolipram used in the previous study was sufficient to induce the significant increase in BDNF mRNA expression relative to the result observed in the present study. However, based on previous preclinical studies, the dose of rolipram in the present study is more consistent with the doses required to achieve antidepressant actions, without unwanted side effects.
Several clinical studies have demonstrated that rolipram has antidepressant efficacy that is equivalent to that of a tricyclic antidepressant (Bertolino et al. 1988; Bobon et al. 1988; Eckmann et al. 1988; Hebenstreit et al. 1989; Scott et al. 1991). Moreover, a recent case report has demonstrated that coadministration of papaverine, nonselective PDE inhibitor, and venlafaxine is effective in the treatment of refractory major depression (Malison et al. 1997). These studies support the hypothesis that a PDE4 inhibitor augments the therapeutic efficacy of antidepressants. In addition, forskolin, a plant disterpene that directly activates adenylyl cyclase, is reported to have antidepressant efficacy in depressed patients (Bersudsky et al. 1996). Taken together, this work supports the hypothesis that activation of the cAMP signal transduction cascade and increased expression of BDNF may be a common target of antidepressant treatment (Duman et al. 1997).
In summary, the results of the present study indicate that repeated coadministration of a PDE4 inhibitor potentiates the induction of BDNF mRNA in response to repeated administration of an antidepressant. The coadministration paradigm shortens the time required for significant induction of BDNF mRNA in response to antidepressant administration. The results suggest that coadministration of a PDE4 inhibitor with an antidepressant may be an efficacious pharmacotherapy for major depression. Further studies to determine the efficacy and safety of repeated coadministration are required to elucidate the potential clinical usefulness of this combination therapy.
References
- Beavo JA, Conti M, Heaslip RJ . (1994): Multiple cyclic nucleotide phosphodiesterases. Mol Pharmacol 46: 399–405
CAS PubMed Google Scholar - Bertolino A, Crippa D, di Dio S, Fichte K, Musmeci G, Porro V, Rapisarda V, Sastre-Y-Hernandez M, Schratzer M . (1988): Rolipram versus imipramine in patients with major, “minor,” or atypical depressive disorder: A double-blind study, double-dummy study aimed at testing a novel therapeutic approach. Int Clin Psychopharmacol 3: 245–253
Article CAS Google Scholar - Bersudsky Y, Kotler M, Shifrin M, Belmaker RH . (1996): A preliminary study of possible psychoactive effects of intravenous forskolin in depressed and schizophrenic patients. J Neural Transm 103: 1463–1467
Article CAS Google Scholar - Bishop JE, Joshi G, Mueller GP, Mouradian MM . (1997): Localization of putative calcium-response regions in the rat BDNF gene. Mol Brain Res 50: 154–160
Article CAS Google Scholar - Bobon D, Breulet M, Gerard-Vandenhove M-A, Guito-Goffioul F, Plomteux G, Satre-Hernandez M, Schratzer M, Troisfontaines B, von Frenckell R, Wachtel H . (1988): Is phosphodiesterase inhibitor a new mechanism of antidepressant action? Eur Arch Psychiatr Neurol Sci 238: 2–6
Article CAS Google Scholar - Condorelli DF, Dell'Albani P, Mudo G, Timmusk T, Belluardo N . (1994): Expression of neurotrophins and their receptors in primary astroglial cultures: Induction by cAMP elevating agents. J Neurochem 63: 509–516
Article CAS Google Scholar - Conti M, Nemoz G, Sette C, Vicini E . (1995): Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr Rev 16: 370–389
Article CAS Google Scholar - Conti M, Jin S-LC . (1999): The molecular biology of cyclic nucleotide phosphodiesterases. Prog Nucleic Acid Res Mol Biol, in press.
- Duman RS, Heninger GR, Nestler EJ . (1997): A molecular and cellular theory of depression. Arch Gen Psychiatry 54: 596–606
Article Google Scholar - Duman RS, Vaidya VA, Nibuya M, Morinobu S, Rydelk-Fitzgerald L . (1995): Stress, antidepressant treatments, and neurotrophic factors: Molecular and cellular mechanisms. The Neuroscientist 1: 351–360
Article CAS Google Scholar - Eckmann F, Fichte K, Meya U, Sastre-Y-Hernandez M . (1988): Rolipram in major depression: Results of a double-blind comparative study with amitriptyline. Curr Ther Res 43: 291–295
Google Scholar - Fleischhacker WW, Hinterhuber H, Bauer H, Pflug B, Berner P, Simhandl C, Wilf R, Gerlach W, Jaklitsch H, Sastre-y-Hernandez M, Schmeding-Wiegel H, Sperner-Unterweger B, Voet B, Schubert H . (1992): A multicenter double-blind study of three different doses of the new cAMP-phosphodiesterase inhibitor rolipram in patients with major depressive disorder. Neuropsychobiology 26: 59–64
Article CAS Google Scholar - Ghosh A, Carnahan J, Greenberg ME . (1994): Requirement for BDNF in activity-dependent survival of cortical neurons. Science 263: 1618–1623
Article CAS Google Scholar - Griebel G, Misslin R, Vogel E, Bourguignon J . (1991): Behavioral effects of rolipram and structurally related compounds in mice: Behavioral sedation of cAMP phosphodiesterase inhibitors. Pharmacol Biochem Behav 39: 321–323
Article CAS Google Scholar - Hebenstreit GF, Fellerer K, Fichte K, Fischer G, Geyer N, Meya U, Sastre-Y-Hernandez M, Schony W, Schratzer M, Soukop W . (1989): Rolipram in major depressive disorder: Results of a double-blind comparative study with imipramine. Pharmacopsychiatry 22: 156–160
Article CAS Google Scholar - Heninger GR, Charney DS . (1987): Mechanisms of action of antidepressant treatments: implications for the etiology and treatment of depressive disorders. In Meltzer HY (ed), Psychopharmacology: The Third Generation of Progress. New York, Raven, pp 535–544
Google Scholar - Kuroda Y, McEwen BS . (1998): Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Mol Brain Res 59: 35–39
Article CAS Google Scholar - Malison RT, Price LH, Nestler EJ, Heninger GR, Duman RS . (1997): Efficacy of papaverine addition for treatment-refractory major depression. Am J Psychiatry 154: 579–580
Article CAS Google Scholar - Mamonous LA, Blue ME, Siuciak JA, Anthony Alter C . (1994): Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci 15: 1929–1939
Google Scholar - Martin-Iverson MT, Todd KG, Alter CA . (1994): Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: Interactions with amphetamine. J Neurosci 14: 1262–1270
Article CAS Google Scholar - Morinobu S, Nibuya M, Duman RS . (1995): Chronic antidepressant treatment down-regulates the induction of c-fos mRNA in response to acute stress in rat frontal cortex. Neuropsychopharmacology 12: 221–228
Article CAS Google Scholar - Nestler EJ, Terwilliger RZ, Duman RS . (1989): Chronic antidepressant administration alters the subcellular distribution of cyclic AMP-dependent protein kinase in rat frontal cortex. J Neurochem 53: 1644–1647
Article CAS Google Scholar - Nibuya M, Morinobu S, Duman RS . (1995): Regulation of BDNF mRNA and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15: 7539–7547
Article CAS Google Scholar - Nibuya M, Nestler EJ, Duman RS . (1996): Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16: 2365–2372
Article CAS Google Scholar - O'Donnell JM . (1993): Antidepressant-like effects of rolipram and other inhibitors of cyclic adenosine monophosphate phosphodiesterase on behavior maintained by differential reinforcement of low response rate. J Pharmacol Exp Ther 264: 1168–1178
CAS PubMed Google Scholar - Ozawa H, Rasenick MM . (1991): Chronic electroconvulsive treatment augments coupling of the GTP-binding protein Gs to the catalytic moiety of adenylyl cyclase in a manner similar to that seen with chronic antidepressant drugs. J Neurochem 56: 330–338
Article CAS Google Scholar - Przegalinski E, Bigajska K . (1983): Antidepressant properties of some phosphodiesterase inhibitors. Pol J Pharmacol Pharm 25: 233–240
Google Scholar - Scott AI, Perini AF, Shering PA, Whalley LJ . (1991): In-patient major depression: is rolipram as effective as amitriptyline? Eur J Clin Pharmacol 40: 127–129
Article CAS Google Scholar - Siuciak JA, Lewis D, Wiegand SJ, Lindsay RM . (1997): Antidepressant-like effect of brain-derived neurotrophic factor. Pharmacol Biochem Behav 56: 131–137
Article CAS Google Scholar - Siuciak JA, Anthony Alter C, Wiegand SJ, Lindsay RM . (1994): Antinociceptive effect of brain-derived neurotrophic factor and neurotrophin-3. Brain Res 633: 326–330
Article CAS Google Scholar - Smith MA, Makino S, Kvetnansky R, Post RM . (1995): Stress alters the express of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 15: 1768–1777
Article CAS Google Scholar - Timmusk T, Palm K, Metsis M, Reintam T, Paalmee V, Sarma M, Persson H . (1993): Multiple promoters direct tissue specific expression of the rat BDNF gene. Neuron 10: 475–489
Article CAS Google Scholar - Ueyama T, Kawai Y, Nemoto K, Sekimoto M, Tone S, Senba E . (1997): Immobilization stress reduced the expression of neurotrophins and their receptors in the rat brain. Neurosci Res 28: 103–110
Article CAS Google Scholar - Wachtel H, Schneider HH . (1986): Rolipram, a novel antidepressant drug, reverses the hypothermia and hypokinesia of monoamine-depleted mice by an action beyond postsynaptic monoamine receptors. Neuropharmacology 25: 1119–1126
Article CAS Google Scholar - Zafra F, Lindholm D, Thoenen H . (1992): Regulation of Brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci 12: 4793–4799
Article CAS Google Scholar - Zafra F, Hengerer B, Thoenen H, Lindholm D . (1990): Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J 11: 3545–3550
Article Google Scholar
Acknowledgements
We thank Solvay-Meiji Pharmaceutical Company for providing rolipram, Hoffmann-LaRoche INC., for providing Ro 20–1724, and Organon Pharmaceutical Company for providing Org 4428. This study was supported by a grant-in-aid for general scientific research from the Ministry of Education, Science, and Culture of Japan and Health Science Research Grants for Research Grants for Research on Brain Science from the Ministry of Health and Welfare of Japan.
Author information
Authors and Affiliations
- Department of Psychiatry, Shiga University of Medical Science, Otsu, Japan
Koichiro Fujimaki MD, Ph.D & Shigeru Morinobu MD, Ph.D - Division of Molecular Psychiatry, Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA
Ronald S Duman Ph.D
Authors
- Koichiro Fujimaki MD, Ph.D
- Shigeru Morinobu MD, Ph.D
- Ronald S Duman Ph.D
Rights and permissions
About this article
Cite this article
Fujimaki, K., Morinobu, S. & Duman, R. Administration of a cAMP Phosphodiesterase 4 Inhibitor Enhances Antidepressant-Induction of BDNF mRNA in Rat Hippocampus.Neuropsychopharmacol 22, 42–51 (2000). https://doi.org/10.1016/S0893-133X(99)00084-6
- Received: 25 March 1999
- Revised: 23 June 1999
- Accepted: 09 July 1999
- Issue Date: 01 January 2000
- DOI: https://doi.org/10.1016/S0893-133X(99)00084-6