Changing partners (original) (raw)
- News & Views
- Published: 23 March 2000
Membrane fusion
Nature volume 404, pages 347–348 (2000)Cite this article
- 220 Accesses
- 11 Citations
- Metrics details
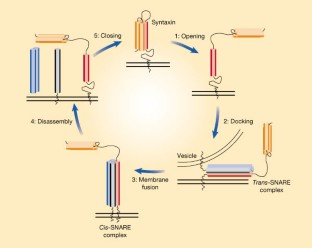
Fusion of the membranes that surround cells and their intracellular organelles occurs during a variety of cellular processes. During neurotransmission, for example, neurons release chemical neurotransmitters from intracellular, membrane-bounded containers (vesicles) into the extracellular space, the synapse. This process involves the merging of the vesicle membrane and the presynaptic plasma membrane, allowing the neurotransmitter to be released. Yet before one membrane can merge with another, a series of protein interactions is required in a process that resembles changing partners at a square dance. On page 355 of this issue1, Misura and colleagues offer new insight into the relationship between two of these partners.
Central to the mechanism of membrane fusion are the SNAREs, which are proteins, found on the target membrane and on the vesicle, that catalyse membrane fusion by assembling into a complex that pins the two fusing membranes together. A key step in the assembly of the SNARE complex is the activation of syntaxin, a name that encompasses a family of SNARE proteins found on target membranes (the presynaptic plasma membrane in the case of neurotrans- mission). Thanks to the resolution of the three-dimensional structure of the core of a SNARE complex2, our understanding of membrane fusion is advancing apace to an atomic level; however, the mechanism by which syntaxin is activated remains poorly understood.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Figure 1: A simplified model for membrane fusion, showing SNARE-complex assembly and conformational changes in syntaxin.
References
- Misura, K. M. S., Scheller, R. H. & Weis, W. I. Nature 404, 355– 362 (2000).
Article ADS CAS Google Scholar - Sutton, R. B., Fasshauer, D., Jahn, R. & Brünger, A. T. Nature 395, 347–353 ( 1998).
Article ADS CAS Google Scholar - Fernandez, I. et al. Cell 94, 841–849 (1998).
Article CAS Google Scholar - Hanson, P. I., Otto, H., Barton, N. & Jahn, R. J. Biol. Chem. 270, 16955–16961 (1997).
Article Google Scholar - Nicholson, K. L. et al. Nature Struct. Biol. 5, 793– 802 (1998).
Article CAS Google Scholar - Söllner, T. et al. Nature 362, 318–324 (1993).
Article ADS Google Scholar - Weber, T. et al. Cell 92, 759–772 (1998).
Article CAS Google Scholar - Hata, Y., Slaughter, C. A. & Südhof, T. C. Nature 366, 347– 366 (1993).
Article ADS CAS Google Scholar - Pevsner, J. et al. Neuron 13, 353–361 (1994).
Article CAS Google Scholar - Carr, C. M., Grote, E., Munson, M., Hughson, F. M. & Novick, P. J. J. Cell. Biol. 146, 333– 344 (1999).
Article CAS Google Scholar - Halachmi, N. & Lev, S. J. Neurochem. 66, 889–897 (1996).
Article CAS Google Scholar - Wu, M. N., Littleton, J. T., Bhat, M. A., Prokop, A. & Bellen, H. J. EMBO J. 17, 127–139 (1998).
Article CAS Google Scholar
Author information
Authors and Affiliations
- Department of Cell Biology, Yale University School of Medicine, 333 Cedar Street, PO Box 208002, New Haven, 06520-8002, Connecticut , USA
Chavela M. Carr & Peter J. Novick
Authors
- Chavela M. Carr
You can also search for this author inPubMed Google Scholar - Peter J. Novick
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toPeter J. Novick.
Rights and permissions
About this article
Cite this article
Carr, C., Novick, P. Changing partners.Nature 404, 347–348 (2000). https://doi.org/10.1038/35006200
- Issue Date: 23 March 2000
- DOI: https://doi.org/10.1038/35006200