Specific activity of cyclin-dependent kinase I is a new potential predictor of tumour recurrence in stage II colon cancer (original) (raw)
Main
Each year >1 million individuals worldwide develop colon cancer with a disease-specific mortality rate of almost 33% (Parkin et al, 2005; Ferlay et al, 2010). Approximately 40% of resected colon cancers are from stage II (T3–4N0M0). The 5-year survival rates vary between 88% in T3N0 patients, and 75% in T4N0 patients. Chemotherapy is widely accepted as adjuvant treatment for stage III patients, whose 5-year survival (stage III A and B) is >75% (Gunderson et al, 2010). Use of chemotherapy for stage II, T4 patients remains controversial despite their worse survival rates. This indicates that the allocation of treatment based solitary on conventional staging methods is not optimal (Kahlenberg et al, 2003; Gunderson et al, 2004; Roukos et al, 2007; Kozak and Moody, 2008; Poston et al, 2008). Over the last decade, there have been important developments towards the discovery of new prognostic and predictive markers that might improve staging methods. The American Society of Clinical Oncology’s Tumor Markers Expert Panel (ASCO TEMP-2006) and its European counterpart, the European Group on Tumor Markers (EGTM-2007) have recently reviewed the literature on these biomarkers. However, all biomarkers reviewed lacked the significant, discriminative value that is required to become implemented into clinical practice (Duffy et al, 2003, 2007; Locker et al, 2006). There is a stringent need for new assays that are able to identify stage II colon cancer patients who might benefit from adjuvant therapy. Genomic instability and altered cell proliferation are major contributors to tumour growth and aggressiveness. Measuring these hallmarks of colon cancer in a quantitative fashion could be a suitable option for risk stratification. The proliferation rate of tumour cells has so far been studied with methods such as 3H-thymidine/BrdU incorporation, mitotic index, or Ki-67/PCNA immunohistochemistry, but none of these tests has reached clinical application (Daidone and Silvestrini, 2001; Michels et al, 2004). Therefore, analysis of the highly conserved drivers of the cell cycle, the cyclin-dependent kinases (CDKs) 1 and 2, may be a more promising approach (Malumbres and Barbacid, 2009). Cyclin-dependent kinase expression is constitutive in tumours but their enzymatic activity changes markedly according to the specific cell-cycle phase. On the molecular level, the activity of CDK is regulated by subunits known as cyclins, and by phosphorylation of conserved tyrosine and threonine residues. Over-expression of cyclins, as well as inactivation of CDK inhibitors, are well documented as prognostic markers for oesophageal, gastric, colorectal, breast, and lung cancer (Gansauge et al, 1997; Sutter et al, 1997; van Diest et al, 1997; Murakami et al, 1999; Sjostrom et al, 2000; Soria et al, 2000; Takano et al, 2000; Takemasa et al, 2000; Korenaga et al, 2002; Peters et al, 2004; Yoshida et al, 2004; Ishihara et al, 2005; Boutros et al, 2007; Suzuki et al, 2007; Begnami et al, 2010; Pereg et al, 2010; Timofeev et al, 2010). However, expression analysis of cyclins and other factors may not necessarily indicate the enzymatic activity of CDKs, which is crucial for the cell-cycle status of the cancer cells. We have recently reported an assay that measures the specific activity (SA) of CDK 1 and CDK2 (Ishihara et al, 2005; Kim et al, 2008; van Nes et al, 2009), based on a well-standardised biochemical assay that requires only small amounts of fresh-frozen tissue and is described in Ishihara et al (2005). The hallmark of this approach is the extraction of functional CDK enzyme from tumour tissue, followed by determination of its kinase activity. We hypothesise that intratumoural kinase activity of CDKs predicts the prognosis of tumour patients with great fidelity, because it directly represents a quantifiable readout for two hallmarks of tumours: increased proliferation and genomic instability. Two large, independent cohorts of breast cancer patients demonstrated that this assay had prognostic value (Kim et al, 2008; van Nes et al, 2009). A CDK-based risk score validated in these studies was a significant and independent prognostic factor, especially for distant recurrence. The aim of this study was to determine the ability of CDK-based analysis to predict recurrence in patients with locally restricted colon cancer. The study was carried out retrospectively on two independent patient cohorts derived from large surgical oncology centres in the Netherlands and Germany. Our results demonstrate that the SA of CDK1 identifies stage II colon cancer patients with a high risk of distant disease recurrence. This patient group may benefit from adjuvant chemotherapy, which would not be recommended according to standard criteria.
Patients and methods
Patients
The study was approved by the local ethics committees at LUMC and TUM. Informed, written consent had been obtained before the study. Fresh-frozen samples of 271 of stage II colon carcinomas were analysed, collected at Leiden University Medical Center (LUMC, 1985–2005), and at Klinikum Rechts der Isar (TUM, 1987–2006). All patients had curative (R0) tumour resection, and none of them received adjuvant or neoadjuvant therapy. Tumour tissue was dissected immediately after resection by a pathologist, snap frozen in liquid nitrogen and stored at −80°C. Development of distant metastasis was observed in 27 patients (11%) after a follow-up of 7.2 years (median). Five samples (1.8%) were excluded due to tumour cell content of <10%. All remaining tissue samples underwent C2P analysis, 12 cases were excluded due to assay failure, or CDK expression level below detection threshold (_n_=3). Of note, all 12 excluded cases were free of tumour recurrence. Hence, 254 samples were available for further analysis (_n_=217 from TUM, and _n_=37 from LUMC).
Determination of CDK-specific activities
In all, 10–20 sections of 100 _μ_m thickness were cut with a cryostat and subjected to CDK analysis. One section of 7 _μ_m thickness was cut from the middle of each block and evaluated by a pathologist after standard H&E staining. Cases with tumour cell content <10% were excluded. The system to measure the CDK-specific activity (CDKSA) is called ‘C2P’ (for ‘cell-cycle profiling’; Sysmex, Kobe, Japan; Ishihara et al, 2005; Kim et al, 2008). In brief, lysates of frozen material were applied to a well of 96-well PVDF filter plate (Millipore, Billerica, MD, USA). Expression of CDKs was detected quantitatively by sequential reactions with primary anti-CDK antibodies, biotinylated anti-rabbit antibodies, and fluorescein-labelled streptavidin. To measure the kinase activity, CDK molecules were immunoprecipitated from the lysate using protein beads, as reported in detail earlier (Ishihara et al, 2005; Kim et al, 2008). Cyclin-dependent kinase SA was calculated as CDK kinase activity units (aU _μ_l–1 lysate) divided by its corresponding CDK expression units (eU _μ_l–1 lysate). Both aU (CDK activity unit) and eU (CDK expression unit) were defined as the expression and activity equivalent to 1 ng of recombinant CDK1 and CDK2, respectively. The distribution of the CDK1SA and CDK2SA within the LUMC and the TUM cohort can be found in Supplementary Figure 1. Further details regarding the quality controls for this assay can be found in the Supplementary data.
Immunofluorescence analysis
Tissue specimens (7 _μ_m) from 207 samples were available for evaluation by immunofluorescence microscopy (Axiovert 200, Zeiss, Göttingen, Germany). After fixation with 3% PFA and antigen retrieval (10 min boiling, sodium citrate buffer, pH=6.0), slides were incubated with anti-Ki-67 antibody (clone MIB-1, M 7240; DAKO, Hamburg, Germany) and/or anti-cytokeratin-20 antibody (rabbit monoclonal, 2039-1, Epitomics, Burlingame, CA, USA) diluted 1:200, followed by incubation with secondary antibodies (Molecular Probes, Darmstadt, Germany; Dianova, Hamburg, Germany), and counterstaining with 4′,6′-diamidino-2-phenylindole (DAPI; Invitrogen, Darmstadt, Germany). Ki-67-positive nuclei from CK20-positive cells were regarded as bona fide tumour cells and were counted in a semi-automated manner using ImageJ freeware (NIH, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/).
Microsatellite instability determination
Tissue from 200 patients of the Munich cohort and all 37 patients of the LUMC was available for DNA isolation with the QIAampDNAMini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNA concentration and quality was checked with an ND-1000 NanoDrop Spectrophotometer (Thermo Fisher, Schwerte, Germany). Subsequently, microsatellite instability (MSI) was tested with the MSI Analysis System, Version 1.2 (Promega, Mannheim, Germany). This assay co-amplifies five mononucleotide repeat markers; BAT-25, BAT-26, NR-21, NR-24, and MONO-27 to determine MSI status. It includes two pentanucleotide repeats, Penta C and D, to make sure that normal and tumour samples are derived from the same patient. The results of this assay have been previously compared with the Bethesda panel markers and proven highly sensitive for MSI determination (Murphy et al, 2006). The MSI status was determined for 32 of the 37 LUMC cases, and for 191 of 200 TUM patients. In 6% of the cases (14 of 237 available DNA samples), MSI status could not be determined based on evaluation of the PCR array data by an experienced pathologist due to ambiguous results.
BRAF
The mutational status of the oncogene BRAF (V600E, GTG>GAG substitution in exon 15) was assessed by high-resolution melting analysis of genomic DNA on a Lightcycler 480 II platform (Roche, Mannheim; SYBR Green I/HRM Dye Protocol), in a modification of published protocols (Pichler et al, 2009). Briefly, 20 ng of genomic DNA (10 ng _μ_l–1) were amplified in total volume of 20 _μ_l with 10 _μ_l High-Resolution Master Mix, 2.4 mM MgCl2, and 0.25 mM each of oligonucleotide primers, 2 _μ_l template DNA and 5.2 _μ_l dH2O. Primer sequences were BRAF Exon 15 For: 5′-GGT GAT TTT GGT CTA GCT ACA G-3′, BRAF Exon 15 Rev: 5′-AGT AAC TCA GCA GCA TCT CAG G-3′. After pre-incubation (95°C, 10 min), amplification of a 147-bp product was carried out in 42 cycles (95°C, 15 s/61°C, 15 s/72°C, 15 s), followed by melting point analysis with an initial phase: 95°C, 5 s, and 72°C, 90 s, followed by a melting profile ranging from 72°C to 95°C in 19.2 min. As a positive control, genomic DNA from the BRAF-mutated colon cancer cell line HT29 was used.
Statistical analysis
Statistical analyses were conducted using R Software version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria). In order to derive optimal cutoff values of quantitative CDK measurements for recurrence risk stratification, maximally selected log-rank statistics have been used. To consider multiple test issue within these analyses, the R-function ‘maxstat.test’ was employed (Hothorn and Zeileis, 2008). To internally validate the derived cutoff, the entire data set was randomly divided in a training and test set (ratio 70 : 30). Furthermore, bootstrap re-sampling analysis was conducted to estimate distribution of derived cutoff values and 95% confidence intervals (CIs), respectively. Multivariable Cox regression was performed to assess recurrence risk differences between derived subgroups in simultaneous consideration of potential confounding factors. Because of the low number of critical events, multivariable regression analyses had to be performed consecutively (one-by-one inclusion of potential confounding factors) to avoid over-adjustment. By the use of survival receiver operating characteristic (ROC) analysis, predictive capability of recurrence risk stratification was assessed cumulatively over the course of the follow-up. In this term, area under the time-dependent ROC curve (concordance index) was reported with 95% bootstrap CI. The Kaplan–Meier methods were used for survival plotting and log-rank test for comparison of survival curves. All statistical tests were conducted two-sided and a _P_-value <0.05 was considered significant.
Results
We have determined the SA of CDK1 and CDK2 (CDK1SA and CDK2SA) in a study population comprised of samples from two independent cohorts of stage II colon cancer patients originating from the Leiden University Medical Centre (LUMC, The Netherlands) and the Klinikum Rechts der Isar, of the Technical University in Munich (TUM, Germany). Five samples (1.8%) were excluded due to tumour cell content of <10%. Twelve cases were excluded due to assay failure, and in three cases the CDK expression levels were below the detection threshold. Of note, all excluded cases were free of tumour recurrence. Altogether, the expression and kinase assay (‘C2P’, in short for ‘cell-cycle profiling’) yielded results in 96% of patients (254 out of 266; _n_=217 from TUM, and _n_=37 from LUMC). There were no statistically significant differences in clinico-pathological characteristics between both cohorts (Table 1). The SA was calculated and indicated as kinase activity in relation to its corresponding mass concentration. The CDK activity unit and CDK expression unit were defined as the equivalent of 1 ng recombinant CDK protein. The distribution of the CDK1SA did not vary significantly between the two study cohorts (Mann–Whitney _U_-test, _P_=0.35), whereas the average of CDK2SA was higher in samples from the Netherlands (_P_=0.012) (Supplementary Figure 1).
Table 1 Patient characteristics
Predictive performance and cutoff derivation of CDKSA for distant recurrence
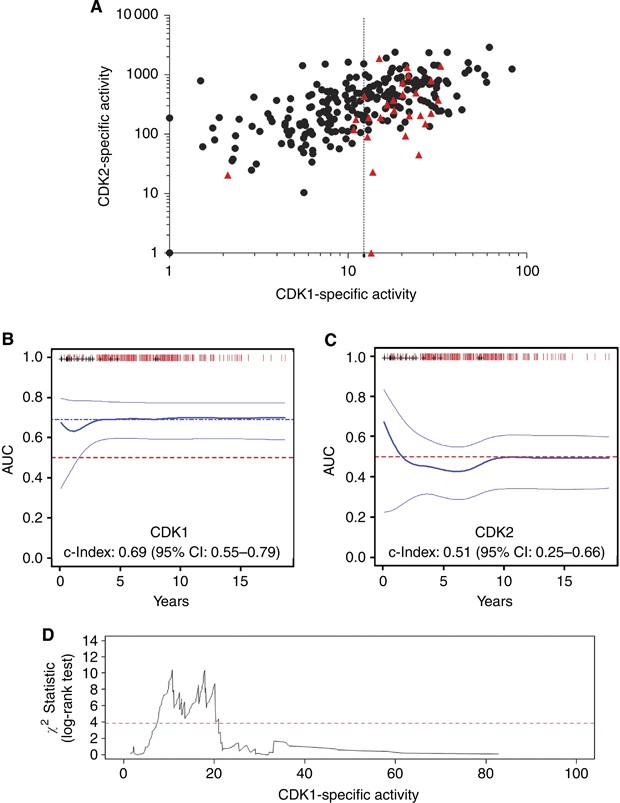
The distribution of clinical samples was plotted on a scatter diagram according to CDK1SA and CDK2SA (Figure 1A). Cases with distant metastasis clustered in the region with high CDK1 activity, suggesting that mainly CDK1SA could have prognostic power. In order to evaluate the prognostic performance of CDK activity for distant metastasis risk, the true positive rates of distant disease recurrence (sensitivity) and corresponding false positive rates (100-specificity) were summarised in a time-dependent ROC curve. The average area under the ROC curve (concordance index or AUC) was 0.69 for CDK1SA (95% CI: 0.55–0.79, _P_=0.024), and 0.51 for CDK2SA, respectively (95% CI: 0.29–0.66, _P_=0.57) (Figures 1B and C). Combined, these results suggested that CDK1SA, but not CDK2SA, is valuable for long-term distant recurrence prediction. Therefore, we focused on CDK1SA and derived the statistically best discriminating cutoff value for CDK1SA, as indicated by maximum log-rank test. For 254 cases, two local maxima of log-rank test statistic were obtained, one for CDK1SA=11 (milli-activity unit per expression unit, maU eU–1), and one for CDK1SA=18 (maU eU–1) (Figure 1D). In order to test the robustness of the selected cutoff values, a second cutoff derivation was performed using the subset of samples with CDK1SA>11 (maU eU–1) (_n_=150). In this analysis, the previously proposed cutoff value of 18 (maU eU–1) neither showed a significant maximum peak, nor was considerably elevated compared with the other candidate cutoff values. This result suggested that the optimal cutoff value for CDK1SA was indeed at 11 (maU eU–1). The final bootstrap analysis confirmed a cutoff value for CDK1SA of 11 (maU eU–1) to be of sufficient discriminant value for further analysis (Supplementary Figure 2). In conclusion, patients with CDK1 activity level >11 (maU eU–1) were classified in the high-risk group (_n_=104, 40% of the patients), and the remaining patients as low-risk group (_n_=150, 60%).
Figure 1
Prognostic performance of the specific activities of CDK1 and CDK2. (A) All cases (_n_=254) plotted on a scatter diagram with logarithmic scales according to CDK1SA and CDK2SA, respectively. Red symbols: patient with distant metastasis, black symbol: no metastasis. (B and C) Time-dependent ROC analysis against CDK1SA (B) or CDK2SA (C). Thick line: concordance index, thin line: 95% CI. Concordance index was 0.69 for CDK1SA (95% CI: 0.55–0.79, _P_=0.036), and 0.51 for CDK2SA (95% CI: 0.25–0.66, _P_=0.57). (D) Derivation of an optimal CDK1SA cutoff value. The maximum log-rank test statistic was obtained when CDK1SA was 11 or 18 (maU eU–1).
CDK1-based risk prediction for distant metastasis-free survival and cause-specific survival
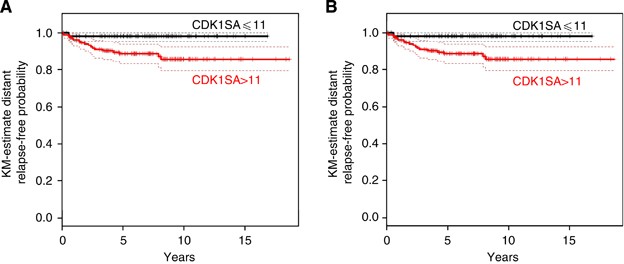
Univariable ‘time-to-event’ analysis showed that patients from the CDK1SA-based low-risk group had significantly longer distant metastasis-free intervals than patients in the high-risk group (hazard ratio (HR)=6.2, 95% CI: 1.45–26.9, _P_=0.0049) (Figure 2A). Importantly, this finding was retained to be statistically significant after adjusting for the multiple log-rank testing, which had been performed in order to obtain the optimal cutoff value of 11 (maU eU–1) (exact conditional Monte-Carlo _P_-value=0.029). The independence of prognostic ability of CDK1SA-based recurrence risk stratification was further evaluated and finally confirmed by multivariable analyses (Table 2). Hazard ratio estimates remained nearly unchanged after consecutive adjustment for the most important clinical-pathological variables, which are currently used for risk evaluation in stage II colon cancer: T4, poor differentiation, presence of obstruction or perforation, lymphatic and vessel invasion, high CEA level, and ⩽12 regional lymph nodes examined (Van Cutsem and Oliveira, 2009) (Table 2). Next, a putative confounding influence of mutations in the BRAF oncogene were analysed. In 217 patients, tissue was available for high-resolution melting analysis of mutations in exon 15 of BRAF. In 32 cases (14.8%), BRAFV600E mutations were detected, 183 patients had BRAF wild-type status, and two cases were not informative. In Kaplan–Meier analysis, the BRAF mutation status was not significantly associated with metastasis-free survival (_P_=0.337), nor with cause-specific survival (_P_=0.253; not shown), and it was excluded as confounding factor for CDK1SA-based risk prediction (Table 2).
Figure 2
Analysis of distant metastasis-free survival and cause-specific survival. (A) Patients classified in the high-risk group (based on CDK1SA >11 maU eU–1) had a significantly worse distant metastasis event rate as compared with the low-risk group (HR=6.2, 95% CI: 1.45–26.9, _P_=0.0049; exact conditional Monte-Carlo _P_-value=0.029). (B) Patients classified in the CDK1SA-based high-risk group had a significantly lower cause-specific survival (HR=7.62, 95% CI: 1.80–32.2, _P_=0.001).
Table 2 Consecutive (one-by-one) adjustment for confounding factors
However, when considering stroma content as adjustment variable, a lack of statistical significance was apparent for the effect of dichotomised CDK1SA. The apparent absence of significance may be explained by the reduced statistical power for this parameter, since about 30% of the cases lacked available stroma content data. Twenty-five patients (10%) died during the follow-up, among them were all 20 patients with distant metastases, and only five patients with no evidence for distant metastases, but with local tumour recurrence. Because of this strong association of distant relapse and death, CDK1SA categorisation was found to be a significant predictor for cause-specific survival (HR high-risk vs low-risk group: 7.62, 95% CI: 1.80–32.2, _P_=0.001) (Figure 2B). This result was thoroughly confirmed in the multivariable analyses. All adjusted estimates of the HR showed values of >7.75, with lower 95% confidence limits >1.80, and _P_-values <0.01. However, a non-significant HR was estimated after adjustment for stroma content (HR high-risk vs low-risk group: 5.22, 95% CI: 0.65–41.5, _P_=0.12).
Correlation between CDKSA, cell proliferation, and microsatellite status
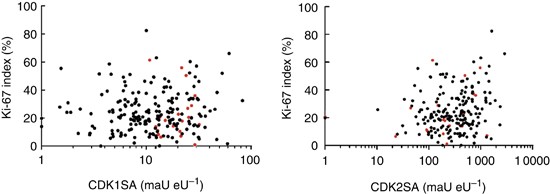
Based on the knowledge of the process of tumourigenesis, high CDK1SA levels could be a reflection of strongly elevated tumour cell proliferation rates. Therefore, we have analysed tumour cell proliferation with the established proliferation marker Ki-67. The Ki-67 labelling index, defined as the percentage of cytokeratin-20-positive cancer cells with Ki-67-positive nuclei, was determined for _n_=207 cases. The median of the Ki-67 index was 21.4%, but it was not retained by Cox regression analysis as significant prognostic factor for distant metastasis (HR=0.69, 95% CI: 0.02–24.0, _P_=0.84). Next, a putative correlation between CDKSAs and the Ki-67 index was examined. However, no significant correlation was found between CDK1SA and Ki-67 index (Spearman’s _ρ_=0.04, _P_=0.54) (Figure 3).
Figure 3
Correlation between CDKSAs and Ki-67 index (percent of Ki-67-positive cells of all CK20-positive tumour cells). Cases were plotted on a scatter diagram according to Ki-67 index against CDK1SA (left), or CDK2SA (right). Red circle: tumour with distant metastasis. Ki-67 showed a weak but significant positive correlation with CDK2SA (Spearman’s _ρ_=0.17, _P_=0.016), but not with CDK1SA (Spearman’s _ρ_=0.04, _P_=0.54).
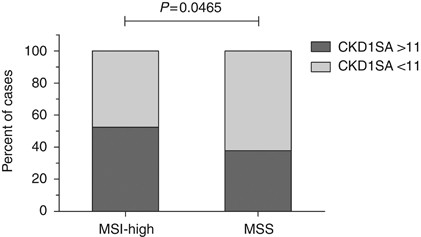
Lastly, a putative correlation between genomic instability and CDK1 activity was tested, since CDKs have been shown to be implicated in cellular responses to genetic instability. Microsatellite instability, caused by defects in the cellular mismatch repair system, has been suggested for colorectal cancer as a favourable prognostic marker. The MSI status was determined with standard methods for 223 cases, and a high level of instability was detected in 59 tumours (26.5%, MSI-High), whereas 164 samples showed stable microsatellite repeats (73%, MSS). Cox regression analysis indicated an estimated five-fold risk-difference regarding distant metastasis-free survival for microsatellite-stable patients, but the results did not attain significance (HR=5.898, 95% CI: 0.782–44.481, _P_=0.085) (Supplementary Figure 3). A significant association of MSI and CDK1SA-based risk stratification was apparent, based on the cutoff for CDK1SA of 11 (maU eU–1). In the patient group with stable microsatellites, significantly more cases with elevated CDK1SA were observed (_χ_2-test, _P_=0.0465; Figure 4). However, a direct comparison of CDK1SA between patients with stable or unstable microsatellites did not attain significance (Supplementary Figure 4).
Figure 4
Association of CDK1SA-based risk stratification with microsatellite-stable phenotype. Among the patients with a stable microsatellite phenotype (MSS), 62% (102 out of 164) were classified in the high-risk group based on CDK1SA. On the other hand, 47.5% (28 out of 59) of the patients with high MSI (MSI-H) were classified as high-risk, based on the CDK1SA threshold (_χ_2-test, _P_=0.0465).
Discussion
This study is the first report demonstrating the SA of CDK1 (CKD1SA) as prognostic biomarker for stage II colon cancer in a blinded and retrospective manner. Two patient cohorts from Germany and the Netherlands were included in this study. Essentially, no differences were observed between these cohorts regarding clinical parameters or CDK1 activity, indicating that the patients were recruited in an unbiased manner. However, the average of CDK2SA was slightly but significantly higher in the samples from the Netherlands (Supplementary Figure 1). This may be due to differences in sample embedding and preparation between the study centres, and to technical variations between the assay systems for CDK1SA and CDK2SA. Previously, CDK1SA- and CDK2SA-based risk was shown to be a clinically useful prognostic marker of early breast cancer of Caucasian and Asian cohorts (Ishihara et al, 2005; Kim et al, 2008; van Nes et al, 2009).
To identify patients with unfavourable prognosis who might benefit from adjuvant chemotherapy, several types of staging systems have been developed (Astler and Coller, 1954; O’Connell et al, 2004; Kozak and Moody, 2008; Gunderson et al, 2010). The current staging systems, however, do not provide accurate risk assessment for stage II patients (O’Connell et al, 2004; Van Cutsem and Oliveira, 2009). Moreover, a number of molecular markers have been proposed, such as mutations in KRAS and TP53, loss of heterozygosity of chromosome 18, and MSI (Fearon and Vogelstein, 1990; Locker et al, 2006; Tejpar et al, 2010). However, none of these candidate biomarkers has yet clearly proven to be useful for diagnosis or staging of patients with stage II colorectal cancer, except for mutations in the BRAF oncogene, which were found to be prognostic for overall survival, particularly in patients with microsatellite-stable tumours (French et al, 2008; Roth et al, 2010). Comprehensive approaches using ‘omics’ technologies have been applied to find biomarkers for colorectal cancer, and we and many others have proposed prognostic transcriptome profile sets so far (Arango et al, 2005; Barrier et al, 2007; Lin et al, 2007; Salazar et al, 2010; Webber et al, 2010). However, inter-patient and even intratumoural heterogeneity, as well as cost factors have precluded wide-scale clinical application. A promising strategy to circumvent tumour heterogeneity is to focus on the central hallmarks of cancer, which are present in almost all tumours irrespective of the underlying molecular changes. Altered cell proliferation and genomic instability are central hallmarks in the case of colon cancer (Hanahan and Weinberg, 2000; Malumbres and Barbacid, 2009). Therefore, we focused on the enzymatic activities and protein expression of CDKs, the main drivers of cell-cycle progression. Moreover, CDK regulators have been well documented as prognostic indicators in many solid tumours (Gansauge et al, 1997; Sutter et al, 1997; van Diest et al, 1997; Murakami et al, 1999; Sjostrom et al, 2000; Soria et al, 2000; Takano et al, 2000; Takemasa et al, 2000; Korenaga et al, 2002; Peters et al, 2004; Yoshida et al, 2004; Ishihara et al, 2005; Boutros et al, 2007; Suzuki et al, 2007; Begnami et al, 2010; Pereg et al, 2010; Timofeev et al, 2010)
Indeed, CDK1SA was a substantial and constant marker for long-term event prediction of distant metastasis in the present study. A robust cutoff value for CDK1SA was derived by choosing a threshold with maximum log-rank statistics (Hothorn and Zeileis, 2008). Importantly, the cutoff value of 11 (maU eU–1) was verified by the adjusted multiple log-rank test. Multivariate analysis retained CDK1SA as independent predictor of distant recurrence. None of the currently accepted clinical risk factors, for example, T4 stage, poor differentiation, obstruction, or tumour perforation (Van Cutsem and Oliveira, 2009), was identified as confounding factor (Table 2). Moreover, CDK1SA was independent of the mutation status in the BRAF oncogene. Therefore, we conclude that CDK1SA-based risk stratification is a reliable prognostic marker for distant metastasis in stage II colon cancer. Two hypotheses, which are not mutually exclusive, may explain the increased intratumoural CDK1SA level in patients with worse prognosis. First, SA of CDK1 may directly reflect higher cancer cell proliferation. To address this question, we have examined a putative correlation between CDK1SA and proliferation. The index of proliferating cancer cells did not significantly correlate with CDK1SA. Moreover, the Ki-67 proliferation index itself was not significant for prognosis, in accordance with earlier findings (Brown and Gatter, 2002). Second, CDK1 activity may be elevated due to chromosomal instability (CIN), a factor already associated with worse prognosis (Walther et al, 2008). Indeed, high CDK1SA levels were significantly correlated with a stable microsatellite phenotype (_χ_2-test, _P_=0.0465). To the best of our knowledge, no reports exist that provide a cause-and-effect link between CDK1 activity and MSI. However, colorectal tumours with stable microsatellites are thought to present CIN, associated with worse prognosis. Thus, microsatellite-stable tumours with high CDK1SA levels in our collective are likely to display chromosomal instability. On the molecular level, regulation of CDK1 activity is orchestrated by cellular checkpoints. Altered expression and activity of the DNA damage and spindle-checkpoint proteins are frequently observed in cancer cells, and contribute to chromosomal instability (Malumbres and Barbacid, 2009). Thus, deregulated checkpoint pathways could cause an aberrant activation of CDK1. Indeed, over-expression of both cyclinB1 and CDC25, important regulators of CDK1 activity, are prognostic markers in colorectal and other cancers (Takemasa et al, 2000; Korenaga et al, 2002; Suzuki et al, 2007). In conclusion, CDK1SA-based analysis is a robust and useful assay to identify patients with a high risk of distant recurrence, who could benefit from adjuvant chemotherapy.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Arango D, Laiho P, Kokko A, Alhopuro P, Sammalkorpi H, Salovaara R, Nicorici D, Hautaniemi S, Alazzouzi H, Mecklin JP, Jarvinen H, Hemminki A, Astola J, Schwartz Jr S, Aaltonen LA (2005) Gene-expression profiling predicts recurrence in Dukes’ C colorectal cancer. Gastroenterology 129: 874–884
Article CAS PubMed Google Scholar - Astler VB, Coller FA (1954) The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg 139: 846–852
Article CAS PubMed PubMed Central Google Scholar - Barrier A, Roser F, Boelle PY, Franc B, Tse C, Brault D, Lacaine F, Houry S, Callard P, Penna C, Debuire B, Flahault A, Dudoit S, Lemoine A (2007) Prognosis of stage II colon cancer by non-neoplastic mucosa gene expression profiling. Oncogene 26: 2642–2648
Article CAS PubMed Google Scholar - Begnami MD, Fregnani JH, Nonogaki S, Soares FA (2010) Evaluation of cell cycle protein expression in gastric cancer: cyclin B1 expression and its prognostic implication. Hum Pathol 41: 1120–1127
Article CAS PubMed Google Scholar - Boutros R, Lobjois V, Ducommun B (2007) CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer 7: 495–507
Article CAS PubMed Google Scholar - Brown DC, Gatter KC (2002) Ki67 protein: the immaculate deception? Histopathology 40: 2–11
Article CAS PubMed Google Scholar - Daidone MG, Silvestrini R (2001) Prognostic and predictive role of proliferation indices in adjuvant therapy of breast cancer. J Natl Cancer Inst Monogr 30: 27–35
Article Google Scholar - Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O (2007) Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer 43: 1348–1360
Article CAS PubMed Google Scholar - Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, Nilsson O, Sturgeon C, Topolcan O (2003) Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer 39: 718–727
Article CAS PubMed Google Scholar - Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61: 759–767
Article CAS PubMed Google Scholar - Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127 (12): 2893–2917
Article CAS PubMed Google Scholar - French AJ, Sargent DJ, Burgart LJ, Foster NR, Kabat BF, Goldberg R, Shepherd L, Windschitl HE, Thibodeau SN (2008) Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res 14: 3408–3415
Article CAS PubMed PubMed Central Google Scholar - Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG (1997) Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res 57: 1634–1637
CAS PubMed Google Scholar - Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK (2010) Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 28: 264–271
Article PubMed Google Scholar - Gunderson LL, Sargent DJ, Tepper JE, Wolmark N, O’Connell MJ, Begovic M, Allmer C, Colangelo L, Smalley SR, Haller DG, Martenson JA, Mayer RJ, Rich TA, Ajani JA, MacDonald JS, Willett CG, Goldberg RM (2004) Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol 22: 1785–1796
Article PubMed Google Scholar - Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100: 57–70
Article CAS PubMed Google Scholar - Hothorn T, Zeileis A (2008) Generalized maximally selected statistics. Biometrics 64: 1263–1269
Article PubMed Google Scholar - Ishihara H, Yoshida T, Kawasaki Y, Kobayashi H, Yamasaki M, Nakayama S, Miki E, Shohmi K, Matsushima T, Tada S, Torikoshi Y, Morita M, Tamura S, Hino Y, Kamiyama J, Sowa Y, Tsuchihashi Y, Yamagishi H, Sakai T (2005) A new cancer diagnostic system based on a CDK profiling technology. Biochim Biophys Acta 1741: 226–233
Article CAS PubMed Google Scholar - Kahlenberg MS, Sullivan JM, Witmer DD, Petrelli NJ (2003) Molecular prognostics in colorectal cancer. Surg Oncol 12: 173–186
Article PubMed Google Scholar - Kim SJ, Nakayama S, Miyoshi Y, Taguchi T, Tamaki Y, Matsushima T, Torikoshi Y, Tanaka S, Yoshida T, Ishihara H, Noguchi S (2008) Determination of the specific activity of CDK1 and CDK2 as a novel prognostic indicator for early breast cancer. Ann Oncol 19: 68–72
Article CAS PubMed Google Scholar - Korenaga D, Takesue F, Yasuda M, Honda M, Nozoe T, Inutsuka S (2002) The relationship between cyclin B1 overexpression and lymph node metastasis in human colorectal cancer. Surgery 131: S114–S120
Article PubMed Google Scholar - Kozak KR, Moody JS (2008) The impact of T and N stage on long-term survival of rectal cancer patients in the community. J Surg Oncol 98: 161–166
Article PubMed Google Scholar - Lin YH, Friederichs J, Black MA, Mages J, Rosenberg R, Guilford PJ, Phillips V, Thompson-Fawcett M, Kasabov N, Toro T, Merrie AE, van Rij A, Yoon HS, McCall JL, Siewert JR, Holzmann B, Reeve AE (2007) Multiple gene expression classifiers from different array platforms predict poor prognosis of colorectal cancer. Clin Cancer Res 13: 498–507
Article CAS PubMed Google Scholar - Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, MacDonald JS, Somerfield MR, Hayes DF, Bast Jr RC (2006) ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 24: 5313–5327
Article CAS PubMed Google Scholar - Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9: 153–166
Article CAS PubMed Google Scholar - Michels JJ, Marnay J, Delozier T, Denoux Y, Chasle J (2004) Proliferative activity in primary breast carcinomas is a salient prognostic factor. Cancer 100: 455–464
Article PubMed Google Scholar - Murakami H, Furihata M, Ohtsuki Y, Ogoshi S (1999) Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Arch 434: 153–158
Article CAS PubMed Google Scholar - Murphy KM, Zhang S, Geiger T, Hafez MJ, Bacher J, Berg KD, Eshleman JR (2006) Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn 8: 305–311
Article CAS PubMed PubMed Central Google Scholar - O’Connell JB, Maggard MA, Ko CY (2004) Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 96: 1420–1425
Article PubMed Google Scholar - Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Article PubMed Google Scholar - Pereg Y, Liu BY, O’Rourke KM, Sagolla M, Dey A, Komuves L, French DM, Dixit VM (2010) Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat Cell Biol 12 (4): 400–406
Article CAS PubMed Google Scholar - Poston GJ, Figueras J, Giuliante F, Nuzzo G, Sobrero AF, Gigot JF, Nordlinger B, Adam R, Gruenberger T, Choti MA, Bilchik AJ, Van Cutsem EJ, Chiang JM, D’Angelica MI (2008) Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol 26: 4828–4833
Article PubMed Google Scholar - Peters MG, Vidal MC, Gimenez L, Mauro L, Armanasco E, Cresta C, Bal de Kier JE, Puricelli L (2004) Prognostic value of cell cycle regulator molecules in surgically resected stage I and II breast cancer. Oncol Rep 12: 1143–1150
CAS PubMed Google Scholar - Pichler M, Balic M, Stadelmeyer E, Ausch C, Wild M, Guelly C, Bauernhofer T, Samonigg H, Hoefler G, Dandachi N (2009) Evaluation of high-resolution melting analysis as a diagnostic tool to detect the BRAF V600E mutation in colorectal tumors. J Mol Diagn 11: 140–147
Article CAS PubMed PubMed Central Google Scholar - Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, Cisar L, Labianca R, Cunningham D, Van Cutsem E, Bosman F (2010) Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 28: 466–474
Article CAS PubMed Google Scholar - Roukos DH, Murray S, Briasoulis E (2007) Molecular genetic tools shape a roadmap towards a more accurate prognostic prediction and personalized management of cancer. Cancer Biol Ther 6: 308–312
Article CAS PubMed Google Scholar - Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, Lopez-Doriga A, Santos C, Marijnen C, Westerga J, Bruin S, Kerr D, Kuppen P, van de Velde CJH, Morreau H, Van Velthuysen L, Glas AM, Van’t Veer LJ, Tollenaar R (2010) Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol 2010: 30
Google Scholar - Sjostrom J, Blomqvist C, Heikkila P, Boguslawski KV, Raisanen-Sokolowski A, Bengtsson NO, Mjaaland I, Malmstrom P, Ostenstadt B, Bergh J, Wist E, Valvere V, Saksela E (2000) Predictive value of p53, mdm-2, p21, and mib-1 for chemotherapy response in advanced breast cancer. Clin Cancer Res 6: 3103–3110
CAS PubMed Google Scholar - Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, Mao L (2000) Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res 60 (15): 4000–4004
CAS PubMed Google Scholar - Sutter T, Doi S, Carnevale KA, Arber N, Weinstein IB (1997) Expression of cyclins D1 and E in human colon adenocarcinomas. J Med 28: 285–309
CAS PubMed Google Scholar - Suzuki T, Urano T, Miki Y, Moriya T, Akahira J, Ishida T, Horie K, Inoue S, Sasano H (2007) Nuclear cyclin B1 in human breast carcinoma as a potent prognostic factor. Cancer Sci 98: 644–651
Article CAS PubMed Google Scholar - Takano Y, Kato Y, van Diest PJ, Masuda M, Mitomi H, Okayasu I (2000) Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases. Am J Pathol 156: 585–594
Article CAS PubMed PubMed Central Google Scholar - Takemasa I, Yamamoto H, Sekimoto M, Ohue M, Noura S, Miyake Y, Matsumoto T, Aihara T, Tomita N, Tamaki Y, Sakita I, Kikkawa N, Matsuura N, Shiozaki H, Monden M (2000) Overexpression of CDC25B phosphatase as a novel marker of poor prognosis of human colorectal carcinoma. Cancer Res 60: 3043–3050
CAS PubMed Google Scholar - Tejpar S, Bertagnolli M, Bosman F, Lenz HJ, Garraway L, Waldman F, Warren R, Bild A, Collins-Brennan D, Hahn H, Harkin DP, Kennedy R, Ilyas M, Morreau H, Proutski V, Swanton C, Tomlinson I, Delorenzi M, Fiocca R, Van Cutsem E, Roth A (2010) Prognostic and predictive biomarkers in resected colon cancer: current status and future perspectives for integrating genomics into biomarker discovery. Oncologist 15: 390–404
Article CAS PubMed PubMed Central Google Scholar - Timofeev O, Cizmecioglu O, Settele F, Kempf T, Hoffmann I (2010) Cdc25 phosphatases are required for timely assembly of CDK1-cyclin B at the G2/M transition. J Biol Chem 285 (22): 16978–16990
Article CAS PubMed PubMed Central Google Scholar - Van Cutsem E, Oliveira J (2009) Primary colon cancer: ESMO clinical recommendations for diagnosis, adjuvant treatment and follow-up. Ann Oncol 20: 49–50
PubMed Google Scholar - Van Diest PJ, Michalides RJ, Jannink L, van der Valk P, Peterse HL, de Jong JS, Meijer CJ, Baak JP (1997) Cyclin D1 expression in invasive breast cancer. Correlations and prognostic value. Am J Pathol 150: 705–711
CAS PubMed PubMed Central Google Scholar - van Nes JG, Smit VT, Putter H, Kuppen PJ, Kim SJ, Daito M, Ding J, Shibayama M, Numada S, Gohda K, Matsushima T, Ishihara H, Noguchi S, van de Velde CJH (2009) Validation study of the prognostic value of cyclin-dependent kinase (CDK)-based risk in Caucasian breast cancer patients. Br J Cancer 100: 494–500
Article CAS PubMed PubMed Central Google Scholar - Walther A, Houlston R, Tomlinson I (2008) Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut 57: 941–950
Article CAS PubMed Google Scholar - Webber EM, Lin JS, Evelyn PW (2010) Oncotype DX tumor gene expression profiling in stage II colon cancer. Application: prognostic, risk prediction. PLoS Curr 2: pii: RRN1177
Article Google Scholar - Yoshida T, Tanaka S, Mogi A, Shitara Y, Kuwano H (2004) The clinical significance of Cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann Oncol 15: 252–256
Article CAS PubMed Google Scholar
Acknowledgements
We thank Alexandra Gnann, Sabine Leis, Gabi van Pelt, and Inès Goossens-Beumer for their excellent technical assistance and for helpful discussion. This study was supported in part by Sysmex Corporation, Hyogo, Japan.
Author information
Author notes
- E C M Zeestraten and M Maak: These authors contributed equally to this work.
Authors and Affiliations
- Department of Surgery, Leiden University Medical Center, Leiden, 2300, The Netherlands
E C M Zeestraten, C J H van de Velde & P J K Kuppen - Department of Surgery, Klinikum Rechts der Isar, Technische Universität München, Ismaninger Str. 22, Munich, 81675, Germany
M Maak, U Nitsche, H Friess, R Rosenberg & K-P Janssen - Sysmex Corporation, Central Research Laboratory, Kobe, 651-2271, Hyogo, Japan
M Shibayama, T Matsushima, S Nakayama, K Gohda & H Ishihara - Institut für Medizinische Statistik und Epidemiologie, Technische Universität München, Munich, 81675, Germany
T Schuster
Authors
- E C M Zeestraten
You can also search for this author inPubMed Google Scholar - M Maak
You can also search for this author inPubMed Google Scholar - M Shibayama
You can also search for this author inPubMed Google Scholar - T Schuster
You can also search for this author inPubMed Google Scholar - U Nitsche
You can also search for this author inPubMed Google Scholar - T Matsushima
You can also search for this author inPubMed Google Scholar - S Nakayama
You can also search for this author inPubMed Google Scholar - K Gohda
You can also search for this author inPubMed Google Scholar - H Friess
You can also search for this author inPubMed Google Scholar - C J H van de Velde
You can also search for this author inPubMed Google Scholar - H Ishihara
You can also search for this author inPubMed Google Scholar - R Rosenberg
You can also search for this author inPubMed Google Scholar - P J K Kuppen
You can also search for this author inPubMed Google Scholar - K-P Janssen
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toK-P Janssen.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Zeestraten, E., Maak, M., Shibayama, M. et al. Specific activity of cyclin-dependent kinase I is a new potential predictor of tumour recurrence in stage II colon cancer.Br J Cancer 106, 133–140 (2012). https://doi.org/10.1038/bjc.2011.504
- Revised: 10 October 2011
- Accepted: 18 October 2011
- Published: 22 November 2011
- Issue Date: 03 January 2012
- DOI: https://doi.org/10.1038/bjc.2011.504