The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia (original) (raw)
Introduction
The nature of the relationship between mood and psychotic illness has been an area of debate in psychiatry since the birth of the specialty. Within the family members of a proband with bipolar disorder (BD), there is an increased risk (above the level in the general population) of unipolar recurrent depression,1 and twin studies show that this is in part explained by shared genetic susceptibility.2 Furthermore, family studies show that schizophrenia is more common than expected within the families of bipolar probands,3, 4, 5 although the risk is less pronounced than for unipolar depression. A recent twin study showed the existence of shared genetic susceptibility to mania and schizophrenia syndromes,6 suggesting that there may also be some shared genetic susceptibility to schizophrenia and BD.
Molecular genetic analysis offers the opportunity to test specific biological genetic hypotheses that can inform our understanding of the nosological relationship between diagnostic categories in general, and the mood and psychotic disorders in particular. A recent meta-analysis of genome-wide association studies on BD 7 provides such an opportunity. A strong association signal was identified within CACNA1C, the gene encoding the α-1C subunit of the L-type voltage-gated calcium channel gene (signal maximal at rs1006737; _P_=7.0 × 10–8). An association signal was present at this polymorphism, with the same risk allele, in each of the three separate component BD samples.7, 8, 9 Within the Wellcome Trust Case Control Consortium (WTCCC)8 BD dataset, the significance level was _P_=7.0 × 10–4.
Here, we have used samples from the UK population recruited and assessed clinically using a methodology similar to that used for our WTCCC BD samples to test the hypotheses that (i) this risk allele is associated with the risk of recurrent major depression, (ii) the risk allele is associated with the risk of schizophrenia.
Methods
Samples
All of the participants in these studies were unrelated, white, living in the British Isles and were of European descent. The protocols and procedures were approved by the relevant ethics review panels where patients were recruited. Cases were excluded if they: (i) had only experienced mood or psychotic illness as a result of alcohol or substance dependence; (ii) had a history of intravenous drug use; (iii) had only experienced mood or psychotic illness secondary to medical illness or medication; or (iv) were biologically related to another study participant. A similar methodology was used for assessment of bipolar, unipolar and schizophrenia cases: a semi-structured lifetime psychiatric interview (Schedules for Clinical Assessment in Neuropsychiatry),10 a review of the available case notes and completion of the Operational Criteria (OPCRIT) checklist of items of psychopathology,11 followed by a consensus best-estimate lifetime diagnosis according to the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV) criteria (American Psychiatric Association 1994).12
BD cases
The 1868 WTCCC BD cases (37% male, mean age 47 (s.d. 13) years, mean age at onset 26 (s.d. 11) years, lifetime experience of psychotic symptoms in 66% and lifetime experience of mood-incongruent psychotic symptoms in 47% of the cases) have been previously described8 and further details of clinical methodology can be found elsewhere.13, 14
Unipolar recurrent major depression cases
In addition to the exclusion criteria described above, unipolar cases were also excluded if they (i) had a first- or second-degree relative with a diagnosis of bipolar affective disorder or schizophrenia, schizotypal disorder, persistent delusional disorder, acute and transient psychotic disorders, or schizoaffective disorder (all of which increase the likelihood of developing BD or a psychotic disorder); or (ii) had ever experienced mood-incongruent psychosis or psychosis outside of mood episodes.
All cases met the DSM-IV12 criteria for recurrent unipolar major depressive disorder (1196 cases, 31% male, mean age 47 (s.d. 12) years, mean age at onset 23 (s.d. 12) years; further information on key variables was available for 348 individuals, 13% of whom had a lifetime occurrence of one or more mood congruent-psychotic features).
Schizophrenia cases
The schizophrenia case sample consisted of 479 unrelated participants meeting the DSM-IV criteria for schizophrenia (68% male, mean age 44.8 (s.d. 13.1) years, mean age at onset 23.8 (s.d. 7.9) years, lifetime episode of mania in 4% and lifetime episode of depression in 23% of the participants). The sample is described elsewhere.15
WTCCC controls
There were 2938 controls recruited from England, Wales and Scotland, who were not screened to exclude the presence of psychiatric illness, and who came from two sources, as described.8
1958 Birth Cohort controls: 1480 controls came from the 1958 Birth Cohort (also known as the National Child Development Study), which includes all births in England, Wales and Scotland during 1 week in 1958. Age at recruitment was 40–49 years; 50% were male.
UK Blood Services controls: The second set of controls was comprised 1458 individuals selected from a sample of blood donors recruited as part of the WTCCC current project. Their age range was 18–69 years; 48% were male.
Expanded reference sample
Consistent with the methodology introduced and described within the WTCCC study,8 an additional source of comparison samples from the UK population was provided by the non-BD disease samples studied within the WTCCC (coronary artery disease, Crohn's disease, hypertension, rheumatoid arthritis, type I and type II diabetes). In total, this sample comprised 11 373 individuals; 49% were male. These samples were not screened for psychiatric illness.
Additional controls typed within the Cardiff laboratory
In addition to the WTCCC controls and expanded reference sample typed within the WTCCC, we used additional UK controls from two sources.
GENESiS controls: (_N_=681, 42% male, mean age 47 (s.d. 9) years) were a subsample of participants who had originally been recruited to the GENESiS (Genetic and Environmental Nature of Emotional States in Siblings) study16 through general practices in England and Wales. GENESiS participants were approached to take part in the current study if they fell into the bottom 20% of the distribution on the Sham Composite Index of Liability to Depression and Anxiety.16 Controls had no current serious medical illness or disability and were screened to exclude a personal or family history of psychiatric illness using a semi-structured telephone interview. We have used controls from this source in previous molecular genetic studies.17
Cardiff controls: (_N_=378, 31% male, mean age 41 (s.d. 15) years) were recruited from two sources. One source was the British Blood Transfusion Service. This sample (_N_=269) was not specifically screened for psychiatric illness, but individuals were not taking regular prescribed medications. In the United Kingdom, blood donors are not remunerated even for expenses and are, therefore, not overrepresented for indigents or the socially disadvantaged, in whom the rate of psychosis might possibly rise above a threshold that would the influence power.18 The second source comprised individuals who were recruited from among those attending family practitioner clinics for non-psychiatric reasons. This sample (_N_=109) was screened to exclude a personal or family history of mood disorder. We have used controls from these two sources extensively in previous molecular genetic studies13, 14 and have not observed evidence for heterogeneity between the control sets nor systematic differences in genetic background from our case samples.13
Genotyping
Affymetrix 500K array
rs1006737 was genotyped as part of the WTCCC genome-wide association study (GWAS)8 in the BD cases, WTCCC controls and in the six other disease cohorts that comprise the expanded reference sample. Further, the schizophrenia cases were genotyped using the same pipeline and at the same time as the WTCCC disease and control samples.15 The GWAS genotyping is described in detail elsewhere.8 All genotyping was undertaken using the GeneChip 500K Mapping Array Set at the Affymetrix service laboratory in San Francisco (CA, USA). Genotype calls were made by members of the WTCCC consortium using the CHIAMO algorithm concurrently with the calling of all the WTCCC disease and control samples.
Further genotyping of rs1006737 was undertaken using alternative genotyping platforms (Sequenom and Amplifluor, San Diego, CA, USA), both to independently verify the genotypes from the Affymetrix assay (using samples typed within WTCCC) and to extend the genotyping to new cases (recurrent unipolar depression) and controls. Unipolar cases (_N_=1196) and GENESiS controls (_N_=681), and subsets of the WTCCC bipolar cases (_N_=1640) and controls (_N_=1351) were genotyped using Sequenom technology. A subset of the unipolar cases (_N_=1043) and GENESiS controls (_N_=418) that had been genotyped by Sequenom were further genotyped by a second independent method, Amplifluor, along with the additional Cardiff controls (_N_=378).
Sequenom genotyping
Individual genotyping of rs1006737 was carried out by Bioserve Biotechnologies Ltd (Laurel, MD, USA) using the Sequenom iPLEX Gold system. All plates for genotyping contained a mixture of cases, controls, blanks and CEU samples to provide a measure of genotyping accuracy. All genotypes were called blind to their sample identity, affection status and genotype calls obtained using other genotyping methods.
Amplifluor genotyping
Allele-specific PCR using the Amplifluor system (Invitrogen Ltd, Paisley, Scotland) was undertaken within the Cardiff laboratory according to the manufacturer's instructions, with alleles being determined by fluorescence polarization measurement using Analyst (LJL Biosystem Ltd, Surrey, England). As for genotyping by Sequenom, DNA plates contained a mixture of cases, controls, blanks and European Caucasian (CEU) samples, and the genotypes were called blind to their sample identity, affection status and genotype calls obtained using other genotyping methods.
Statistical analysis
Genotype call concordance across platforms was assessed. Departure from Hardy–Weinberg equilibrium was tested using a _χ_2 goodness-of-fit test. Contingency tables were used to test genotypes and alleles for differences between sample sets and, where relevant, to calculate odds ratios and their 95% confidence intervals. Cochran–Armitage trend tests were performed using Graphpad Instat (www.graphpad.com). As we were interested in the disease-predisposing effect of a specific allele, the hypothesis tests included the direction of effect and we therefore report one-tailed tests, except where specified.
Consistent with our previous phenotypic analyses of the WTCCC BD dataset,19 we undertook an exploratory analysis using logistic regression to seek one or more phenotype variables that showed an enhanced signal at the risk allele. In the bipolar sample, we tested the following clinical variables: age at onset of illness, lifetime occurrence of psychosis, Bipolar Affective Disorder Dimension Scale (BADDS)20 mania score, BADDS depression score, BADDS psychosis score, BADDS incongruence score, lifetime occurrence of suicidal ideation, lifetime occurrence of rapid cycling and polarity of onset of illness. In the unipolar sample, we tested the following clinical variables: age at onset, lifetime occurrence of psychosis, BADDS depression score, Global Assessment Scale (GAS) score during the worst episode and lifetime occurrence of suicidal behavior. In the schizophrenia sample, we tested the following clinical variables: age at onset, lifetime occurrence of a major depressive episode, lifetime occurrence of a manic episode and lifetime occurrence of suicidal ideation.
Results
Genotyping quality was excellent, with consistency between assays
Affymetrix 500K array: Call rate=99.9%; Hardy-Weinberg Equilibrium (HWE) _P_-value, _P_>0.05.
Sequenom: Overall genotype call rate=99.3%; HWE _P_-value, GENESiS controls, _P_>0.05. Genotyping concordance: three genotype discrepancies were observed in 2911 genotypes (1321 controls, 1590 bipolar cases) genotyped by both Sequenom and Affymetrix (concordance rate =99.89%).
Amplifluor: Overall genotype call rate=99.8%; HWE _P_-value GENESiS controls and Cardiff controls, _P_>0.05. Genotyping concordance: one genotype discrepancy was observed in 1315 genotypes (938 unipolar cases, 377 controls) genotyped by both Amplifluor and Sequenom (concordance rate=99.92%).
Genotype distributions at rs1006737 were closely similar in all control and reference sample sets
The genotype distributions at rs1006737 were closely similar in each of the different control and reference samples, and there were no significant differences between any pair-wise comparisons of these control and reference groups (Supplementary Table 1). The two additional GENESiS and Cardiff blood donor control sample sets that were genotyped (that is, those control data independent of the WTCCC) were, thus, combined to form a single independent control group against which the unipolar cases could be compared.
The BD risk allele at rs1006737 was significantly more common in recurrent depression than in controls or reference samples
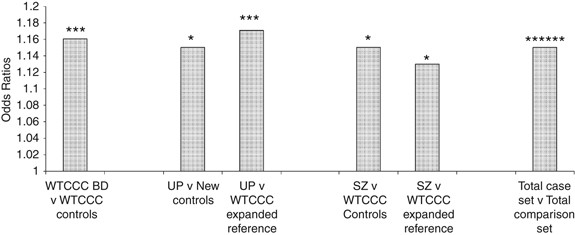
Comparison of the recurrent unipolar depression cases with the independent new controls showed significant overrepresentation of the BD risk allele, A (minor allele), in cases compared with controls (_P_=0.013). Similarly, comparison with the WTCCC expanded reference sample showed significant overrepresentation of the A allele in cases with an increased level of statistical significance (_P_=3.9 × 10–4) because of the larger sample size of the reference sample. See Table 1 and Figure 1.
Table 1 Genotype and allele distributions and _P_-values in controls, bipolar, schizophrenia and unipolar disorder cases for the CACNA1C polymorphism rs1006737
Figure 1
Odd ratios for CACNA1C polymorphism rs1006737 for cases versus control groups. *P<0.01; ***P<0.001; ******P<10–6. UP, unipolar recurrent major depression cases; new controls, GENESiS controls and Cardiff controls combined; SZ, schizophrenia cases; total case set, combined UK bipolar disorder cases, unipolar recurrent major depression cases and schizophrenia cases; total comparison set, combined WTCCC controls, GENESiS controls and Cardiff controls.
BD risk allele at rs1006737 was significantly more common in schizophrenia than controls
Comparison of the schizophrenia cases against the WTCCC controls (as used in the original study design15) showed significant overrepresentation of the A allele in cases compared with controls (_P_=0.034). Similarly, comparison against the WTCCC expanded reference sample showed significant overrepresentation of that allele in cases (_P_=0.043). See Table 1 and Figure 1.
Genotype and allele distributions at rs1006737 did not differ significantly between BD, schizophrenia and recurrent unipolar depression
Comparison of the genotype and allele distributions in the three different case samples (BD, schizophrenia and recurrent unipolar depression) did not show significant differences (_χ_2 analysis of 3 × 3 and 3 × 2 contingency tables, _P_>0.05).
Exploratory genotype–phenotype analyses using the risk allele at rs1006737
In the exploratory phenotypic analysis of the BD sample, of the variables examined, only BADDS depression score was retained within the logistic regression model (_P_=0.016). Comparing the allele distributions of bipolar cases with BADDS depression score >59 and those scoring <59 showed that the risk A allele was significantly associated with depression score >59 (_P_=0.007, corresponding clinically to experiencing at least one episode of major depression that at least meets the International Classification of Diseases (ICD10)22 criteria for ‘severe’ major depression). Within the unipolar and schizophrenia samples the logistic model did not identify any variables that were retained within logistic regression models at P<0.05.
Discussion
We have found that, within our white UK case–control samples, the BD risk A allele at rs1006737 within the CACNA1C gene is also associated with risk of recurrent major depression and schizophrenia. The effect sizes were similar for all diagnostic categories tested, and we found no statistically significant differences between the genotype or allele distributions across the three case sets, although each differed significantly from the reference (control) individuals.
This association signal had been identified at a genome-wide significance level (_P_=7.0 × 10–8) in a meta-analysis of 4387 BD cases and 6209 controls7 with evidence for association coming from each of the component datasets within the meta-analysis. The significance level for the BD case–control analysis within the original WTCCC study was _P_=7.0 × 10–4 (two-tailed). It is of interest to note that, if we were to combine the UK bipolar, recurrent depression and schizophrenia cases into one composite mood-psychosis case set (_N_=3509) and compare the genotype distribution at rs1006737 with the total UK comparison set (_N_=15 316), the significance of association would be substantially strengthened (by more than three orders of magnitude) above that seen in our UK case–control BD sample (_P_=3.2 × 10–7, two-tailed), see Figure 1. If we use the meta-analysis of the three BD datasets within the Ferreira et al. paper7 (of which one is the WTCCC BD sample) and the new data for unipolar depression and schizophrenia, again the statistical significance for association is strengthened (this time by more than an order of magnitude, _P_=1.25 × 10−9) compared with that in the BD meta-analyses reported in the Ferreira et al. paper7 (_P_=7.0 × 10−8, Supplementary Table 2).
A strength of our study is that we have used cases ascertained from the UK mental health services and assessed using a similar clinical methodology, which includes a semi-structured lifetime psychiatric interview and review of case records. In addition, we have access to a very large reference set of the UK individuals, which has been genotyped for the polymorphism of interest and which shows closely similar genotype distributions across the component comparison sample sets (be they blood donor controls, the 1958 Birth Cohort or non-psychiatric disease samples). A further strength is that the polymorphism of interest has excellent genotyping quality in the assays used, with a demonstrated high degree of genotype concordance across platforms, providing confidence in comparing and combining data across platforms.
Most of the reference individuals used in our study were not screened to exclude psychiatric illness. This will tend to reduce the power to identify differences from cases. Although this is known to have minimal effect for phenotypes with a lifetime prevalence of around 1% (such as BD and schizophrenia),18 it may be important for phenotypes, such as depression, that are more common. Despite this possible reduction in power for our association analysis of depression, in our study, the comparison of recurrent depression cases with the reference samples was significant and the genotype distributions were closely similar between those controls that had been screened to exclude psychiatric illness (GENESiS) and the other (unscreened) controls and reference samples.
It has been hypothesized that some genes will influence the risk for relatively specific domains of psychopathology, whereas others will have a more general influence on the risk.5, 23 At the level of diagnostic category, our data did not allow us to discriminate between our BD, unipolar depression and schizophrenia case sets, suggesting that this locus contributes a relatively general increase of risk across the mood-psychosis clinical spectrum.
The only variable that was retained in the exploratory genotype–phenotype logistic regression analysis within the bipolar case set was the BADDS depression score, with increasing score being more associated with the risk allele. This raises the possibility that this locus may particularly influence the depressive aspects of illness. In other words, it may be that this locus primarily influences susceptibility to depression, irrespective of the current diagnostic category used to classify the individual's overall clinical picture. This hypothesis will require testing in independent, preferably large, BD samples, as well as in other samples from across the mood-psychosis spectrum. Given the similar effect sizes observed within the samples studied here, it can be expected that a systematic study of very large samples—preferably across an even broader spectra of neuropsychiatric illness—may be necessary to refine the phenotypic expression at this polymorphism. It is interesting to note that the recently reported Genetic Association Information Network (GAIN) consortium study of recurrent depression reported nominally significant evidence for association at CACNA1C,24 although it is important to recognize that the genotyping platform (and hence, single nucleotide polymorphism set) differed from that used in the WTCCC analysis. CACNA1C has not been highlighted among the top hits in schizophrenia GWAS reported to date. Publicly available schizophrenia GWAS data are currently very sparse and, given that the expectation would be that association signals at CACNA1C would be modest (and, hence, not among the top hits),25 it will be important for systematic meta-analysis and mega-analysis to be undertaken across all available datasets.
It can be expected that the early robust associations that will emerge from GWAS meta-analyses will be biased towards loci that have a relatively broad spectrum of phenotypic effect. The reason is that, with our current reliance on diagnostic categories that have a wide range of possible clinical picture, it is inevitable that there will be substantial variation between the clinical spectrum of cases represented in samples assembled by different researchers. For example, important systematic variation may occur in potentially important variables such as severity, treatment response, symptom profile, ‘comorbidity’ with other clinical syndromes, functional impairment, familial loading, exposure or environmental triggers, and so on.26 Thus, the loci that are likely to emerge most readily from such samples are those that confer risk across a broad phenotypic spectrum. Identification of loci conferring a more specific phenotypic risk is likely to require different approaches, such as phenotype refinement19 and the use of multiple, large, well-characterized samples.27, 28
In summary, within our UK sample ascertained and studied clinically using similar methodologies, we have found that the same risk allele at CACNA1C increases risk to categorically defined BD, schizophrenia and recurrent major depression. This is a clear demonstration that there is an overlap in the biological underpinnings of susceptibility to mental illness across the clinical spectrum of mood and psychotic disorders. In addition to contributing to a better understanding of the pathogenesis of major psychiatric illness, such knowledge will be useful in shaping psychiatric nosology to move the field from reliance on a diagnostic and classification system that is based only on clinical description and towards a scheme that better reflects the underlying biology of the psychiatric entities encountered in our clinics. This will be of great benefit to patients.
References
- McGuffin P, Katz R . The genetics of depression and manic-depressive disorder. Br J Psychiatry 1989; 155: 294–304.
Article CAS Google Scholar - McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A . The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 2003; 60: 497–502.
Article Google Scholar - Berrettini WH . Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry 2000; 48: 531–538.
Article CAS Google Scholar - Bramon E, Sham PC . The common genetic liability between schizophrenia and bipolar disorder: a review. Curr Psychiatry Rep 2001; 3: 332–337.
Article CAS Google Scholar - Craddock N, Owen MJ . The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry 2005; 186: 364–366.
Article Google Scholar - Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P . A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry 2002; 159: 539–545.
Article Google Scholar - Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008; 40: 1056–1058.
Article CAS Google Scholar - Wellcome Trust Case Control Consortium. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature 2007; 447: 661–678.
Article Google Scholar - Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K et al. Whole-genome association study of bipolar disorder. Mol Psychiatry 2008; 13: 558–569.
Article CAS Google Scholar - Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R et al. SCAN. Schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry 1990; 47: 589–593.
Article CAS Google Scholar - McGuffin P, Farmer A, Harvey I . A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48: 764–770.
Article CAS Google Scholar - American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Press: Washington, DC, 1994, pp 886.
- Green EK, Raybould R, Macgregor S, Gordon-Smith K, Heron J, Hyde S et al. Operation of the schizophrenia susceptibility gene, neuregulin 1, across traditional diagnostic boundaries to increase risk for bipolar disorder. Arch Gen Psychiatry 2005; 62: 642–648.
Article CAS Google Scholar - Green EK, Raybould R, Macgregor S, Hyde S, Young AH, O'Donovan MC et al. Genetic variation of brain-derived neurotrophic factor (BDNF) in bipolar disorder: case-control study of over 3000 individuals from the UK. Br J Psychiatry 2006; 188: 21–25.
Article Google Scholar - O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 2008; 40: 1053–1055.
Article CAS Google Scholar - Sham PC, Sterne A, Purcell S, Cherny S, Webster M, Rijsdijk F et al. GENESiS: creating a composite index of the vulnerability to anxiety and depression in a community-based sample of siblings. Twin Res 2000; 3: 316–322.
CAS PubMed Google Scholar - Green EK, Grozeva D, Raybould R, Elvidge G, Macgregor S, Craig I et al. P2RX7—a Bipolar and Unipolar Disorder candidate susceptibility gene?. Am J Med Genet B Neuropsychiatr Genet 2009; published online 21 January 2009; PMID 19160446.
- Moskvina V, Holmans P, Schmidt KM, Craddock N . Design of case-controls studies with unscreened controls. Ann Hum Genet 2005; 69: 566–576.
Article CAS Google Scholar - Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D et al. Strong genetic evidence for a selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype. Mol Psychiatry 2008; PMID 19078961.
- Craddock N, Jones I, Kirov G, Jones L . The Bipolar Affective Disorder Dimension Scale (BADDS)—a dimensional scale for rating lifetime psychopathology in bipolar spectrum disorders. BMC Psychiatry 2004; 4: 19–28.
Article Google Scholar - Endicott J, Spitzer RL, Fleiss JL, Cohen J . The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 33: 766–771.
Article CAS Google Scholar - WHO. The International Classification of Diseases 10 Classification of Mental and Behavioural Disorders Diagnostic Criteria for Research. World Health Organisation: Geneva, 1993, pp 248.
- Craddock N, O'Donovan MC, Owen MJ . Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder and mixed (or ‘schizoaffective’) psychoses. Schizophr Bull 2009; 35: 482–490, Advance online March 2009.
Article Google Scholar - Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T et al. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry 2008; 14: 359–375.
Article Google Scholar - Craddock N, O'Donovan MC, Owen MJ . Genome-wide association studies in psychiatry: lessons from early studies of non-psychiatric and psychiatric phenotypes. Mol Psychiatry 2008; 13 (7): 649–653.
Article CAS Google Scholar - Craddock N, O'Donovan MC, Owen MJ . Phenotypic and genetic complexity of psychosis. Invited commentary on … Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry 2007; 190: 200–203.
Article Google Scholar - Psychiatric GWAS Consortium Steering Committee. A framework for interpreting genome-wide association studies of psychiatric disorders. Mol Psychiatry 2009; 14: 10–17.
Article Google Scholar - Cross Disorder Phenotype Group of the Psychiatric GWAS Consortium. Dissecting the phenotype in genome-wide association studies of psychiatric illness. Br J Psychiatry 2009; 195: 97–99.
Article Google Scholar
Acknowledgements
We are indebted to all individuals who have participated in, or helped with, our research. Funding for sample collection was provided by grants from the Wellcome Trust and Medical Research Council. Funding for genotyping was provided by grants from the Wellcome Trust. We acknowledge and thank all those involved in the Wellcome Trust Case Control Consortium (WTCCC).
Author information
Author notes
- The members of the Wellcome Trust Case Control Consortium (WTCCC) are listed in Supplementary Online Material.
Authors and Affiliations
- Department of Psychological Medicine and Neurology, School of Medicine, Cardiff University, Cardiff, UK
E K Green, D Grozeva, I Jones, G Kirov, K Gordon-Smith, C Fraser, L Forty, E Russell, M L Hamshere, V Moskvina, I Nikolov, P A Holmans, M J Owen, M C O'Donovan & N Craddock - Department of Psychiatry, National Centre for Mental Health, University of Birmingham, Birmingham, UK
L Jones, S Caesar & K Gordon-Smith - Biostatistics and Bioinformatics Unit, School of Medicine, Cardiff University, Cardiff, UK
M L Hamshere, V Moskvina, I Nikolov & P A Holmans - SGDP, The Institute of Psychiatry, King's College London, Denmark Hill, London, UK
A Farmer & P McGuffin
Authors
- E K Green
You can also search for this author inPubMed Google Scholar - D Grozeva
You can also search for this author inPubMed Google Scholar - I Jones
You can also search for this author inPubMed Google Scholar - L Jones
You can also search for this author inPubMed Google Scholar - G Kirov
You can also search for this author inPubMed Google Scholar - S Caesar
You can also search for this author inPubMed Google Scholar - K Gordon-Smith
You can also search for this author inPubMed Google Scholar - C Fraser
You can also search for this author inPubMed Google Scholar - L Forty
You can also search for this author inPubMed Google Scholar - E Russell
You can also search for this author inPubMed Google Scholar - M L Hamshere
You can also search for this author inPubMed Google Scholar - V Moskvina
You can also search for this author inPubMed Google Scholar - I Nikolov
You can also search for this author inPubMed Google Scholar - A Farmer
You can also search for this author inPubMed Google Scholar - P McGuffin
You can also search for this author inPubMed Google Scholar - P A Holmans
You can also search for this author inPubMed Google Scholar - M J Owen
You can also search for this author inPubMed Google Scholar - M C O'Donovan
You can also search for this author inPubMed Google Scholar - N Craddock
You can also search for this author inPubMed Google Scholar
Consortia
Wellcome Trust Case Control Consortium
Corresponding author
Correspondence toN Craddock.
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Green, E., Grozeva, D., Jones, I. et al. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia.Mol Psychiatry 15, 1016–1022 (2010). https://doi.org/10.1038/mp.2009.49
- Received: 15 January 2009
- Revised: 31 March 2009
- Accepted: 03 April 2009
- Published: 21 July 2009
- Issue Date: October 2010
- DOI: https://doi.org/10.1038/mp.2009.49