Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint (original) (raw)
Main
The metaphase-to-anaphase transition is induced by APC — the ubiquitin protein ligase involved in ubiquitination and degradation of Pds1 and cyclin B — resulting in anaphase onset and exit from mitosis, respectively1. APC coupled with the activator Cdc20 targets Pds1 and cyclin B, whereas the APC–Cdh1 complex recognizes cyclin B (ref. 2). APC is only activated by Cdc20 after all chromosomes have established bipolar attachment to the mitotic spindle. The presence of even a single unattached kinetochore triggers the spindle checkpoint to inhibit anaphase onset3. The spindle checkpoint proteins Mad1, Mad2, BubR1 (Mad3 in yeast), Bub1, Bub3 and Mps1 localize to kinetochores during mitosis4,5. Before anaphase, Cdc20 is prevented from activating the APC by binding with a checkpoint complex containing Mad2, BubR1 and Bub3 (refs 6,7,8,9,10). Unattached kinetochores seem to enucleate the complex formation11,12,13. Thus, Cdc20 is kept inactive until all kinetochores attach to the spindle.
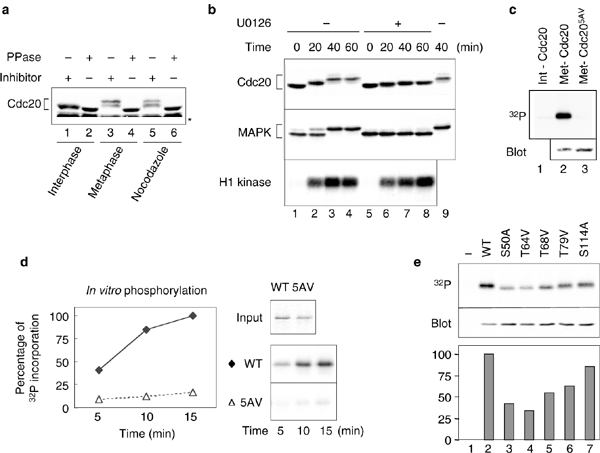
Cdc20 undergoes phosphorylation during mitosis and is a substrate for Cdc2 in vitro14. Phosphorylation of Cdc20 is not required for the activation of APC in human cells14,15. We examined the phosphorylation state of Cdc20 in three types of X. laevis egg extracts: interphase, metaphase and spindle checkpoint extracts. Mature eggs are arrested at metaphase II by cytostatic factor (CSF)16. The cytoplasmic extract of these eggs is arrested at metaphase, advancing into interphase after addition of calcium17. The CSF-arrested extract reproduces the spindle checkpoint after incubation with a high density of sperm nuclei and the microtubule inhibitor nocodazole18. Immunoblot analysis of the extracts demonstrates a slower electrophoretic mobility of Cdc20 in metaphase and spindle checkpoint extracts, compared with that in interphase extracts (Fig. 1a). This mobility shift was caused by phosphorylation, as phosphatase treatment greatly enhanced the mobilty of Cdc20 (Fig. 1a).
Figure 1: MAP kinase contributes to phosphorylation of Cdc20 during mitosis.
(a) Cdc20 is phosphorylated during mitosis. Interphase (lanes 1 and 2), metaphase (lanes 3 and 4) and spindle checkpoint extracts (lanes 5 and 6) were incubated with phosphatase inhibitors (lanes 1, 3 and 5) or λ protein phosphatase (lanes 2, 4 and 6), followed by immunoblotting with anti-Cdc20 antibody. The asterisk indicates cross-reacting proteins. (b) Phosphorylation of Cdc20 is reduced after inhibiting MAPK. Immunoblots of interphase extracts driven into mitosis were prepared by the addition of non-degradable cyclin B for the times indicated in the absence (lanes 1–4) or presence of U0126 (lanes 5–8). The blots were probed with anti-Cdc20 (top) or anti-MAPK (middle) antibodies. A duplicate of lane 3 is shown in lane 9 for comparison of mobility. An autoradiograph of histone H1 kinase assays as a measure of Cdc2 activity (bottom). (c) Labelling of Cdc20 and Cdc205AV in egg extracts with 32P. The autoradiograph (top) shows wild-type Cdc20 labelled in interphase extract (lane 1) or in metaphase extract (lane 2) and Cdc205AV labelled in metaphase extract (lane 3). Immunoblot analysis of Cdc20 and Cdc205AV at interphase (bottom). (d) MAPK phosphorylates recombinant Cdc20 in vitro. Right panels show Coomassie blue staining of recombinant proteins used as the substrates (top, input). Autoradiographs of Cdc20 phosphorylated for times indicated are shown (bottom). The graph shows relative incorporation of 32P. (e) Labelling of Cdc20 and Cdc20 single mutants in metaphase extract with 32P. Interphase extracts containing no Cdc20 (lane 1) or various Cdc20 proteins, as indicated, were immunoblotted for Cdc20 (middle) or driven into mitosis in the presence of γ-32P-ATP, followed by immunoprecipitation of Cdc20. Autoradiograph of 32P incorporation (top). The graph shows the relative incorporation of 32P normalized with the protein levels from the immunoblot analysis, with wild-type designated as 100%.
Cdc20 contains several Ser/Thr-Pro sequences that are potential targets for Cdc2 and MAPK, which is important for the spindle checkpoint18,19,20. Furthermore, active MAPK is enriched at kinetochores during mitosis21,22. To determine if MAPK contributes to Cdc20 phosphorylation, we attempted to perform MAPK immunodepletion but were only able to remove 80% of MAPK. As an alternative approach, we used the MAPK kinase (MEK) inhibitor U0126 to block MAPK activation. This inhibitor reduced the mobility shift of both MAPK and Cdc20 in the mitotic extract, despite the fact that Cdc2 was fully active (Fig. 1b). This result demonstrates that Cdc2 does not account for all of the phosphorylation on Cdc20 and that the MAPK pathway is also involved in phosphorylation of Cdc20.
Seven Ser/Thr-Pro sequences in X. laevis Cdc20 (Ser 50, Thr 64, Thr 68, Thr 79, Ser 114, Thr 165 and Ser 461) are conserved in human Cdc20, with the first five sites residing within the Mad2-binding region13. We generated a mutant, Cdc205AV, by substituting Ala for Ser 50 and Ser 114 and Val for Thr 64, Thr 68 and Thr 79. We found that wild-type Cdc20 could be labelled with 32P, whereas Cdc205AV incorporated very little 32P in egg extracts (Fig. 1c), indicating that the mutations prevent mitotic phosphorylation. Furthermore, we found that recombinant Cdc20 was a substrate for MAPK in vitro, but Cdc205AV was not (Fig. 1d). Next, we generated mutants with each of the five Ser or Thr residues mutated individually to Ala or Val, respectively. Labelling of these proteins with 32P in mitotic extracts demonstrated a significant reduction of 32P incorporation into each of the single mutants, except for Cdc20S114A (Fig. 1e), suggesting that Ser 50, Thr 64, Thr 68 and Thr 79 are the phosphorylation sites.
Wild-type Cdc20 labelled with 32P in metaphase extract was subjected to two-dimensional tryptic phosphopeptide analysis, which revealed three major phosphopeptides (labelled 1, 2 and 3 in Fig. 2a). Peptide 3 disappeared and a new peptide (4) emerged in Cdc20 labelled in U0126-treated extract (Fig. 2a), indicating that peptide 3 contains the MAPK phosphorylation site. Peptide mapping of the five single mutants demonstrated that peptide 3 was undetectable in Cdc20T64V and Cdc20T68V mutants (Fig. 2b). The map of Cdc20T64V produced another distinct phosphopeptide (5) that probably contains phosphorylated Thr 68. Thus, peptide 3 may contain both Thr 64 and Thr 68 and peptide 4 (that resulted from U0126 treatment) may contain phosphorylated Thr 64 or Thr 68. These results suggest that MAPK phosphorylates either Thr 64 or Thr 68. Peptides 1 and 2 contain Ser 50 and Thr 79, as they disappeared in the Cdc20S50A and Cdc20T79V mutants, respectively (Fig. 2b). The map of Cdc20S114A (Fig. 2b) was essentially identical to that of the wild-type Cdc20, consistent with the notion that Ser 114 is not a mitotic phosphorylation site.
Figure 2: Phosphopeptide analysis of Cdc20.
(a) Inhibition of MAPK affects the phosphopeptide map. Wild-type Cdc20 was labelled in metaphase extract with 32P in the absence (left) or presence (right) of U0126. Cdc20 was then immunoprecipitated and processed for two-dimensional tryptic phosphopeptide mapping. (b) Phosphopeptide maps of various Cdc20 mutants. Wild-type and Cdc20 single mutants were labelled with 32P in metaphase extracts and processed for tryptic phosphopeptide mapping. Peptides 1, 2 and 3 contain phosphorylation at Ser50, Thr79 and Thr64–Thr68, respectively. Inhibition of MAPK affects peptide 3.
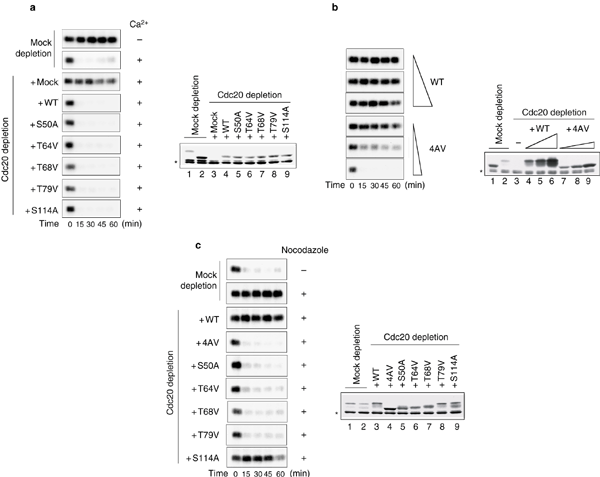
As previously demonstrated23, CSF-arrested extract depleted for Cdc20 maintained the Cdc2-associated histone H1 kinase activity after addition of calcium to inactivate CSF (Fig. 3a). Thus, APC cannot target cyclin B in the absence of Cdc20. In Cdc20-depleted extracts supplemented with Cdc20 or Cdc20 single mutants to the level of endogenous Cdc20, calcium addition inactivated Cdc2 with kinetics similar to that in mock-depleted extracts, indicating that phosphorylation-deficient Cdc20 mutants are functional.
Figure 3: Function of Cdc20 phosphorylarion.
(a) Cdc20 mutants are able to inactivate Cdc2. Calcium chloride was added to induce mitotic exit in mock-depleted extracts or Cdc20-depleted extracts supplemented with mock or various Cdc20 proteins, as indicated on the left. Samples were taken immediately before the addition of calcium chloride and every 15 min thereafter for the measurement of Cdc2-associated histone H1 kinase activity. Autoradiographs of histone H1 phosphorylation are shown. Samples at 60min were also immunoblotted for Cdc20 (right). (b) An elevated level of Cdc204AV abolishes the CSF-mediated metaphase arrest. CSF-arrested extracts were immunodepleted for endogenous Cdc20 and supplemented with increasing concentrations of Cdc20 or Cdc204AV, as indicated. Samples were incubated without calcium chloride and aliquots were taken every 15 min for histone H1 kinase assays. Immunoblotting of Cdc20 was performed (right) with samples at 1h, as well as mock-depleted extracts at interphase (lane 1) or metaphase (lane 2) and Cdc20-depleted extracts (lane 3). Wild-type Cdc20 was added to fourfold, eightfold and 16-fold of the endogenous level and Cdc204AV to 0.8-, 1.6- and 3.2-fold of the endogenous level. The slightly slower mobility of translated Cdc20 when compared with that of endogenous protein is caused by additional amino acids inserted at the amino terminus of the protein from the cloning vector. (c) Phosphorylation-deficient Cdc20 mutants are insensitive to the spindle checkpoint signal. Sperm nuclei and nocodazole were incubated with extracts that were mock-depleted, or depleted for the endogenous Cdc20 and supplemented with mock or various Cdc20 proteins, as indicated. Calcium chloride was then added and Cdc2 activity was measured. Autoradiographs of histone H1 phosphorylation (left) and immunoblots of Cdc20 in the samples at time 0 (right) are shown. The asterisks indicate cross-reacting proteins.
We also tested the function of Cdc204AV (S50A, T64V, T68V, T79V). Interestingly, a threefold excess of Cdc204AV triggered inactivation of Cdc2 within 15 min of its addition, even in the absence of calcium (Fig. 3b). This indicates that the extract escaped CSF-induced metaphase arrest. At similar or higher levels, wild-type Cdc20 had no effect on the CSF arrest (Fig. 3b). These results indicate that Cdc20 is a downstream target of CSF and that phosphorylation of Cdc20 renders it more sensitive to the anaphase-inhibitory signal produced by the CSF.
Next, we determined whether Cdc20 phosphorylation is involved in regulation of the spindle checkpoint. After incubation with sperm nuclei and nocodazole, the mock-depleted extract and extracts containing Cdc20 or Cdc20S114A sustained Cdc2 activity after calcium addition, indicative of a functional spindle checkpoint (Fig. 3c). However, Cdc2 activity declined rapidly in extracts containing any of the four phosphorylation-deficient single mutants or Cdc204AV (Fig. 3c). Consistent with Cdc2 inactivation, chromosomes were decondensed (data not shown), indicating that these extracts failed to maintain a mitotic arrest in response to unattached kinetochores. These results demonstrate that phosphorylation-deficient Cdc20 mutants are insensitive to the spindle checkpoint signal and that phosphorylation of Cdc20 at all of the mitotic phosphorylation sites is required for it to be inhibited by the spindle checkpoint.
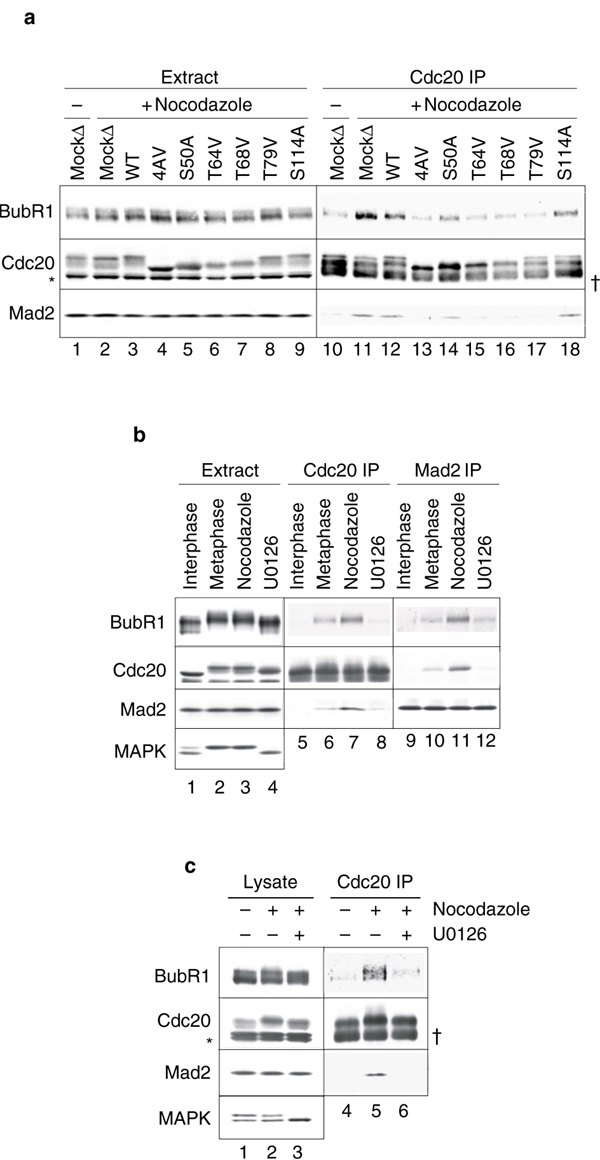
To determine whether the checkpoint proteins could associate with phosphorylation-deficient Cdc20 mutants, we immunoprecipitated Cdc20 from extracts containing various Cdc20 proteins and then performed immunoblot analysis. Interaction of Cdc20 with the checkpoint proteins was enhanced after checkpoint activation in mock-depleted extract when compared with that in metaphase extracts without nocodazole treatment (Fig. 4a, lanes 10 and 11). A similar interaction was observed with Cdc20 added into extracts depleted for the endogenous Cdc20 (Fig. 4a, lane 12). However, the levels of both Mad2 and BubR1 were greatly reduced in Cdc204AV immunoprecipitates (Fig. 4a, lane 13) and moderately reduced with Cdc20 single mutants Cdc20S50A, Cdc20T64V, Cdc20T68V and Cdc20T79V (Fig. 4a, lanes 14–17). Consistent with the finding that Ser 114 is not a mitotic phosphorylation site (Fig. 2b), the S114A mutation did not affect association with checkpoint proteins (Fig. 4a, lane 18). These results demonstrate that phosphorylation of Cdc20 at Ser 50, Thr 64, Thr 68 and Thr 79 is important for its association with BubR1 and Mad2 in response to unattached kinetochores.
Figure 4: Phosphorylation of Cdc20 facilitates its association with spindle checkpoint proteins.
(a) Cdc20 phosphorylation mutants are compromised in their ability to interact with Mad2 and BubR1. Spindle checkpoint was induced in mock-depleted extracts (lanes 2 and 11) and Cdc20-depleted extracts supplemented with wild-type Cdc20 (lanes 3 and 12), Cdc204AV (lanes 4 and 13), or each of the single mutants (lanes 5–9 and 14–18), as indicated. Lanes 1 and 10, mock-depleted extracts kept at metaphase. The extracts were subjected to Cdc20 immunoprecipitation (lanes 10–18). Proteins in the extracts (lanes 1–9) or associated with Cdc20 immunoprecipitates were immunoblotted for BubR1, Cdc20 and Mad2, as indicated. (b) Inhibition of the MAPK pathway reduces the interaction between Cdc20 and spindle checkpoint proteins in egg extracts. Cdc20 (lanes 5–8) or Mad2 (lanes 9–12) were immunoprecipitated from interphase (lane 1), metaphase (lane 2) and checkpoint-induced extracts in the absence of U0126 treatment (lane 3) or from checkpoint-induced extract treated with U0126 (lanes 4). Extracts or immunoprecipitates were immunoblotted for BubR1, Cdc20, Mad2 and MAPK as indicated. (c) Inhibition of the MAPK pathway reduces the interaction between Cdc20 and the spindle checkpoint proteins in XTC cells. Cell lysates were prepared from asynchronous XTC cells (lanes 1 and 4) or nocodazole-arrested cells that were treated with DMSO (lanes 2 and 5) or with U0126 (lanes 3 and 6). The lysates were then subjected to Cdc20 immunoprecipitation. The lysates (lanes 1–3) or immunoprecipitates (lanes 4–6) were immunoblotted with the indicated antibodies. The asterisk indicates cross-reacting proteins and the dagger the immunoglobulin heavy chain.
As MAPK probably phosphorylates Cdc20 at Thr 64 or Thr 68 (Fig. 2), we asked whether inhibition of MAPK by U0126 also affected the interaction between Cdc20 and the spindle checkpoint proteins. The amount of Mad2 and BubR1 associated with Cdc20 decreased in U0126-treated extract when compared with that in untreated extract (Fig. 4b, lanes 7 and 8). Similarly, U0126 treatment reduced the amount of Cdc20 and BubR1 in Mad2 immunoprecipitates in response to unattached kinetochores (Fig. 4b, lanes 11 and 12). Furthermore, U0126 treatment of cultured Xenopus laevis tadpole cells (XTC) also reduced the nocodazole-induced interaction between Cdc20, BubR1 and Mad2 (Fig. 4c). Inhibition of MAPK with a structurally unrelated inhibitor PD98059 gave the same result (see Supplementary Information, Fig. S1), indicating that the effect is unlikely to be caused by a non-specific target of the inhibitors. These results demonstrate that MAPK activity is important for the interaction of Cdc20 with the spindle checkpoint proteins in both egg extracts and somatic cells. It remains to be determined whether the site of Cdc20 phosphorylation in somatic cells is the same as that in egg extracts.
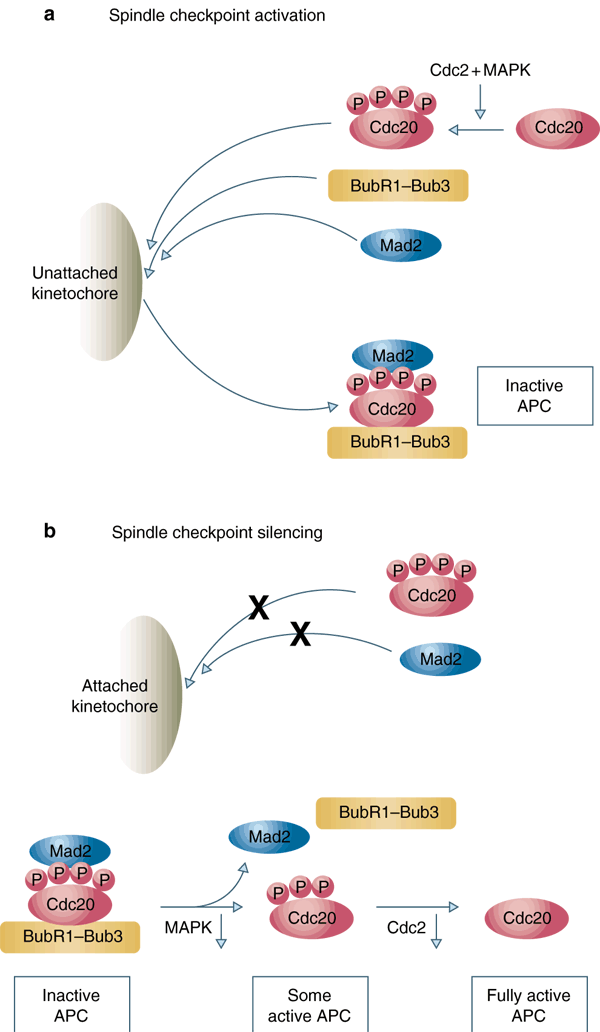
Here, we demonstrate that phosphorylation of X. laevis Cdc20 at Ser 50, Thr 64, Thr 68 and Thr 79 is required for binding of Cdc20 and inhibition by the spindle checkpoint proteins. Similarly to wild-type Cdc20, a phosphorylation-mimicking Cdc204D mutant (with the phosphorylation sites altered to Asp) interacts poorly with the spindle checkpoint proteins in the absence of checkpoint activation, and the interaction is stimulated by unattached kinetochores (see Supplementary Information, Fig. S2). This result suggests that Cdc204D is unable to constitutively associate with spindle checkpoint proteins, supporting the notion that unattached kinetochores are important for the formation of the complex between the checkpoint proteins and fully phosphorylated Cdc20 (Fig. 5a). Cdc204D fails to bypass the requirement of MAPK in the spindle checkpoint (see Supplementary Information, Fig. S3), suggesting that MAPK may have other targets in the spindle checkpoint. Another spindle checkpoint protein CENP-E is also a substrate for MAPK (ref. 22), but the functional significance of this phosphorylation has not been determined.
Figure 5: The function of Cdc20 phosphorylation in activating and terminating the spindle checkpoint.
(a) Unattached kinetochores recruit and activate Mad2, BubR1 and Bub3 during spindle checkpoint arrest, allowing their association with Cdc20 that has been phosphorylated by Cdc2 and MAPK. Binding of Mad2, BubR1 and Bub3 to Cdc20 prevents Cdc20 from activating the APC. (b) Attached kinetochore does not recruit Mad2 and Cdc20, preventing the generation of new Mad2–BubR1–Bub3–Cdc20 complex. The existing checkpoint complex may be disassembled through dephosphorylation of Cdc20. Inactivation of MAPK by an unknown mechanism may result in initial dephosphorylation of Cdc20 and partial activation of the APC. The APC–Cdc20 then triggers the first phase of cyclin B degradation and inactivation of Cdc2, resulting in further dephosphorylation of Cdc20 and rapid accumulation of active APC–Cdc20 to trigger the anaphase.
As a functional spindle checkpoint is dependant on fully phosphorylated Cdc20, loss of phosphorylation at any one of these sites will destabilize the checkpoint complex and allow Cdc20 to activate APC. Inactivation of MAPK towards the end of mitosis may allow accumulation of a small amount of APC–Cdc20 that contributes to the first phase of cyclin B destruction24. The initial Cdc2 inactivation then results in further dephosphorylation of Cdc20 and consequently a rapid accumulation of Cdc20 that is free of spindle checkpoint proteins (Fig. 5b). Thus, dephosphorylation of Cdc20 at multiple MAPK and Cdc2 sites may provide a mechanism to disassemble the existing spindle checkpoint complex. It may also function to quickly silence the spindle checkpoint signal after the initial dephosphorylation of Cdc20, resulting in the switch-like fashion of the metaphase-to-anaphase transition.
Methods
X. laevis egg extracts, spindle checkpoint assay, immunoblot analysis and immunodepletion.
CSF-arrested extracts and demembranated sperm nuclei were prepared as described17. To prepare interphase extracts, the CSF-arrested extracts were driven into interphase by incubation with 0.5 mM calcium chloride for 30 min at 23 °C and another 30-min incubation in the presence of 100 μg ml−1 cycloheximide to prevent resynthesis of cyclin B and mitotic entry. The checkpoint extracts were prepared in CSF-arrested extract by incubation with sperm nuclei (15,000 μl−1) at 23 °C for 10 min and another 20-min incubation with 10 μg ml−1 nocodazole. H1 kinase assay, immunoblot analysis and immunodepletion were performed as described25,26. Immunoprecipitation of Cdc20 was performed as described12.
Synthesis of Cdc20.
In vitro transcription and translation of Cdc20 in egg extracts were performed as described26. To prevent resynthesis of endogenous Cdc20, 100 μg ml−1 cycloheximide was added to Cdc20-depleted extracts in all experiments that involved replacement of endogenous Cdc20 with translated Cdc20 or various Cdc20 mutants. Mutations in CDC20 were generated using QuikChange (Stratagene, La Jolla, CA).
Phosphatase treatment.
For controls with phosphatase inhibitors, extracts were diluted 30-fold in λ phosphatase reaction buffer (New England Biolabs, Beverly, MA) supplemented with 1× phosphatase inhibitor cocktail set II (Calbiochem, La Jolla, CA) and 0.2 μM microcystin-LR (Calbiochem, La Jolla, CA). For phosphatase treatment, extracts were incubated in phosphatase reaction buffer containing 2 mM magnesium chloride and 200U μl−1 λ protein phosphatase (New England Biolabs, Beverly, MA) at 30 °C for 30 min.
Labelling with 32P.
Translations of various Cdc20 were incubated in 20 μl of Cdc20-depleted interphase extracts at 23 °C for 1 h to remove any mitotic phosphorylation. The extracts were then driven into metaphase by the addition of an equal volume of Cdc20-depleted metaphase extracts containing 150 μCi γ-32P-ATP in the absence or presence of U0126 (Upstate Biotechnology, Lake Placid, NY). After incubation for 30 min, the samples were diluted tenfold with RIPA buffer (10 mM Tris at pH 7.2, 150 mM sodium chloride, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 0.2 μM microcystin-LR and 10 μg ml−1 each of leupeptin, pepstatin and chymostatin (LPC)) and spun at 13,000_g_ for 5 min. The supernatants were subjected to anti-Cdc20 immunoprecipitation by mixing at 4 °C for 1 h with anti-Cdc20 antibodies pre-bound to Affi-Prep Protein A beads (Bio-Rad, Hercules, CA). The immunoprecipitates were washed twice with RIPA, twice with RIPA containing 0.5 M sodium chloride and twice with RIPA. The samples were resolved by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and processed for autoradiography. The amount of 32P incorporated was analysed by Phosphorimager scanning and quantified with ImageQuant software (Version 1.2, Amersham Biosciences, Piscataway, NJ).
In vitro phosphorylation of Cdc20.
Wild-type or mutant X. laevis CDC20 cDNA was cloned into pQE9 vector (Qiagen, Valencia, CA) and the hexahistidine-tagged protein was produced in bacteria. The inclusion bodies containing recombinant Cdc20 were prepared and the proteins were purified from SDS–PAGE gels as described25 and concentrated in 10 mM Hepes and 1 mM magnesium chloride. Immunoprecipitates of MAPK from CSF-arrested extracts were washed twice with lysis buffer (10 mM potassium phosphate at pH 7.5, 1 mM EDTA, 5 mM EGTA, 50 mM β-glycerophosphate, 1 mM sodium vanadate, 0.5% Triton X-100, 0.1% sodium deoxycholate, 1 mM magnesium chloride, 2 mM DTT and LPC), twice with lysis buffer containing 0.5 M sodium chloride, twice with lysis buffer and once with kinase buffer (20 mM Hepes at pH 7.8, 10 mM magnesium chloride, 0.1 mg ml−1 bovine serum albumin and 3 mM β-mercaptoethanol). Kinase reactions were performed by incubating MAPK immunoprecipitates at 30 °C in 10 μl kinase buffer containing 100 μM ATP, 2 μCi γ-32P-ATP and 1 μg of the recombinant Cdc20 proteins as substrates. Reactions were stopped after 5, 10 and 15 min of incubation by the addition of SDS–PAGE sample buffer.
Two-dimensional tryptic phosphopeptide mapping.
Wild-type or mutant Cdc20 proteins were labelled with 32P as described above, except that the labelling was performed in 100 μl extracts with 450 μCi of γ-32P-ATP. For Fig. 2a, U0126 was added to 400 μM during interphase to inhibit MAPK and the control sample was incubated with the same volume of dimethyl sulphoxide (DMSO). The samples were diluted with 0.5 ml RIPA buffer and immunoprecipitated for 1 h with Affi-Prep Protein A beads pre-blocked with unlabelled CSF-arrested extract and coated with anti-Cdc20 antibodies. The immunoprecipitates were resolved by SDS–PAGE and the Cdc20 proteins labelled with 32P were excised from the gel for two-dimensional tryptic phosphopeptide mapping as described27.
XTC cell culture and immunoprecipitation.
XTC cells were maintained at room temperature (22–23 °C) in 70% Leibovitz's L-15 medium supplemented with 10% foetal bovine serum and penicillin/streptomycin (Invitrogen, Carlsbad, CA). Cells were synchronized at the G1–S phase boundary by double-thymidine block. The first block was imposed for 24 h with 2 mM thymidine (Sigma, St. Louis, MO) in the culturing medium. The cells were then released from the block by washing twice with serum-free medium and grown in the culturing medium for 12 h before the second addition of thymidine. After 24 h, cells were released from the block in the culturing medium containing 100 ng ml−1 nocodazole. After 6 h, some cells were treated with 10 μM U0126, whereas others received equal volumes of DMSO as a control. To ensure a mitotic arrest, cells were also treated with 10 μM MG-132 (Calbiochem, La Jolla, CA), a proteasome inhibitor. In the absence of MG-132, cells escaped nocodazole-induced mitotic arrest in the presence of U0126 (E. C. and R.-H. C., unpublished results). After 5 h, mitotic cells were collected by gentle shake-off and cell pellets were lysed in lysis buffer containing 0.2 μM Microcystin-LR. The protein concentration of the lysates was quantified using the DC Protein Assay system (BioRad, Hercules, CA) and adjusted to the same concentration. For Fig. 4c, 95 μl of the lysates at 17 mg ml−1 were used for immunoprecipitation at 4 °C for 1 h with anti-Cdc20 antibody. The immunoprecipitates were washed twice with lysis buffer, twice with lysis buffer containing 0.5 M sodium chloride and once with lysis buffer. The immunoprecipitates and 40 μg of the lysates were resolved by SDS–PAGE for immunoblot analysis.
Note: Supplementary Information is available on the Nature Cell Biology website.
References
- Page, A.M. & Hieter, P. The anaphase-promoting complex: new subunits and regulators. Annu. Rev. Biochem. 68, 583–609 (1999).
Article CAS Google Scholar - Visintin, R., Prinz, S. & Amon, A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 (1997).
Article CAS Google Scholar - Amon, A. The spindle checkpoint. Curr. Opin Genet. Dev. 9, 69–75 (1999).
Article CAS Google Scholar - Shah, J.V. & Cleveland, D.W. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell 103, 997–1000 (2000).
Article CAS Google Scholar - Musacchio, A. & Hardwick, K.G. The spindle checkpoint: structural insights into dynamic signalling. Nature Rev. Mol. Cell Biol. 3, 731–741 (2002).
Article CAS Google Scholar - Fang, G., Yu, H. & Kirschner, M.W. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12, 1871–1883 (1998).
Article CAS Google Scholar - Hwang, L.H. et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279, 1041–1044 (1998).
Article CAS Google Scholar - Kim, S.H., Lin, D.P., Matsumoto, S., Kitazono, A. & Matsumoto, T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Article CAS Google Scholar - Tang, Z., Bharadwaj, R., Li, B. & Yu, H. Mad2-Independent inhibition of APC–Cdc20 by the mitotic checkpoint protein BubR1. Dev. Cell 1, 227–237 (2001).
Article CAS Google Scholar - Sudakin, V., Chan, G.K. & Yen, T.J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).
Article CAS Google Scholar - Chen, R.H. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158, 487–496 (2002).
Article CAS Google Scholar - Chung, E. & Chen, R.H. Spindle Checkpoint Requires Mad1-bound and Mad1-free Mad2. Mol. Biol. Cell. 13, 1501–1511 (2002).
Article CAS Google Scholar - Zhang, Y. & Lees, E. Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: model for spindle checkpoint regulation. Mol Cell. Biol 21, 5190–5199 (2001).
Article CAS Google Scholar - Kramer, E.R., Scheuringer, N., Podtelejnikov, A.V., Mann, M. & Peters, J.M. Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell 11, 1555–1569 (2000).
Article CAS Google Scholar - Yudkovsky, Y., Shteinberg, M., Listovsky, T., Brandeis, M. & Hershko, A. Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem. Biophys Res. Commun. 271, 299–304 (2000).
Article CAS Google Scholar - Masui, Y. & Markert, C.L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 177, 129–145 (1971).
Article CAS Google Scholar - Murray, A.W. Cell Cycle Extracts. Meth. Cell Biol. 36, 573–597 (1991).
Google Scholar - Minshull, J., Sun, H., Tonks, N.K. & Murray, A.W. MAP-kinase dependent mitotic feedback arrest in Xenopus egg extracts. Cell 79, 475–486 (1994).
Article CAS Google Scholar - Takenaka, K., Gotoh, Y. & Nishida, E. MAP kinase is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J. Cell Biol. 136, 1091–1097 (1997).
Article CAS Google Scholar - Wang, X.M., Y, Zhai. & Ferrell. J. Jr, A role for mitogen-activated protein kinase in the spindle assembly checkpoint in XTC cells. J. Cell Biol. 137, 433–443 (1997).
Article CAS Google Scholar - Shapiro, P.S. et al. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J. Cell Biol. 142, 1533–1545 (1998).
Article CAS Google Scholar - Zecevic, M. et al. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J. Cell Biol. 142, 1547–1558 (1998).
Article CAS Google Scholar - Lorca, T. et al. Fizzy is required for activation of the APC/cyclosome in Xenopus egg extracts. EMBO J 17, 3565–3575 (1998).
Article CAS Google Scholar - Raff, J.W., Jeffers, K. & Huang, J.Y. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J. Cell Biol. 157, 1139–1149 (2002).
Article CAS Google Scholar - Chen, R.H., Shevchenko, A., Mann, M. & Murray, A.W. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 143, 283–295 (1998).
Article CAS Google Scholar - Sharp-Baker, H. & Chen, R.H. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J. Cell Biol. 153, 1239–1250 (2001).
Article CAS Google Scholar - Boyle, W.J., Van der Geer, P. & Hunter, T. Phoshphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Meth. Enzymol. 201, 110–149 (1991).
Article CAS Google Scholar
Acknowledgements
We thank M. Goldberg and T. Huffaker for critically reading the manuscript. This work was supported by grants from the National Institutes of Health and the David and Lucile Packard Foundation to R.-H.C.
Author information
Authors and Affiliations
- Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853, USA
Eunah Chung & Rey-Huei Chen
Authors
- Eunah Chung
You can also search for this author inPubMed Google Scholar - Rey-Huei Chen
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toRey-Huei Chen.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Chung, E., Chen, RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint.Nat Cell Biol 5, 748–753 (2003). https://doi.org/10.1038/ncb1022
- Received: 13 July 2002
- Accepted: 09 May 2003
- Published: 13 July 2003
- Issue Date: 01 August 2003
- DOI: https://doi.org/10.1038/ncb1022