The autophagosomal–lysosomal compartment in programmed cell death (original) (raw)
Diversity of programmed cell death: morphological evidence of autophagic cell death in states of health and disease
In the last decade apoptosis attracted growing interest of the scientific community and a tremendous gain in knowledge concerning the molecular events of its signalling, preparation and execution has been achieved (for review:1,2,3,4,5,6). However, accumulating morphological and biochemical evidence suggests that programmed cell death (PCD) is not confined to apoptosis but that cells use different pathways for active self-destruction: condensation prominent, type I or apoptosis; autophagy prominent, type II PCD etc.1,7,8,9,10,11,12 In particular, according to the original morphological and histochemical based description of apoptosis, the autophagosomal–lysosomal system was considered not to play a role in initial stages of apoptosis.13,14 Rather, the action of lysosomes appeared to be restricted to the (heterophagic) degradation of apoptotic bodies ensuing after phagocytosis by vital cells.13,14 Thus, phagocytosis of apoptotic cell residues constitutes an integral part of the overall suicide process,5 the molecular aspects of which are reviewed elsewhere in this issue of Cell Death and Differentiation. In the present paper, first morphological, functional and molecular features of autophagic cell death (type II PCD) will be reviewed. Secondly, recent evidence indicating an important function of lysosomal cysteine proteases in the preparatory stages of apoptosis (type I PCD) such as processing of BID and caspases will be addressed.
Reviewing the literature revealed a non-consistent use of terms to describe cell death associated with autophagocytosis as it includes necrosis, non-apoptotic type of cell death, apoptosis/type I PCD, autophagic cell death/type II PCD and others (Table 1).15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38 For the purpose of summarising the morphological evidence of its occurrence in states of health and disease, electronmicroscopical demonstration of autophagic vacuoles in dying cells was taken as conditio sine qua non to denote cell death as autophagic/type II PCD; in addition, available histo- and biochemical criteria indicating a role of the autophagosomal–lysosomal compartment were included into Table 1. It should be emphasised, that referring to the morphological/histochemical features does not imply a causative relationship between macroautophagocytosis and eventual manifestation of a cell's suicide; data suggesting a functional link between these phenomena as well as related molecular events will be discussed in subsequent paragraphs.
Table 1 Occurrence of autophagic cell death–in vivo (examples)
Autophagic cell death appears to be a phylogenetically old phenomenon as it has been observed in the slime mold Dictyostelium discoideum and in the nematode C elegans (Table 1);15,16,17 it even might have developed before apoptosis.10,39 A large body of morphological, histo- and biochemical evidence for autophagic cell death in vivo was provided by developmental biology. Insect metamorphosis may be considered as one of the most extreme biological conditions of tissue remodelling including autophagic cell death; cells of ecto-, endo and mesodermal origin are affected.7,9,11,18,19,20,21,22,23 Likewise, in vertebrate development, autophagic cell death appears to be a prominent feature associated with organ morphogenesis as exemplified by shaping of extremities, cavity formation in intestine and regression of sexual anlagen.7,8,11,25 Autophagic cell death also has been reported to occur in adulthood of insects and vertebrates including humans; it is often associated with the elimination of (large secretory) cells during adjustment of sexual organs and ancillary tissues to seasonal reproduction.7,8,11,26,28,31 As to pathophysiology, autophagic cell death has been associated with experimental and human neurological diseases32,33,34,35 as well as cytotoxic drug treatment.38
In summary, without attempting to force the data, the morphological observations strongly support the concept that autophagic cell death is characterised by features different from apoptosis (Figure 1) and which is a phenomenon of general importance occurring in a broad spectrum of (patho)physiological conditions. The most prominent features of autophagic cell death comprise degradation of cytoplasmic components resulting in progressive loss of electrondensity; the descriptions of autophagic cell death consistently include that degradation of cytoplasmic components precede nuclear collapse. Notably, the number of mitochondria in the cytoplasm decreased but those present in cytoplasm appeared intact; conceivably, the remaining mitochondria maintain ATP-levels required for the completion of autodigestion (see below and Figure 2). Like apoptosis, autophagic cell death has been described to be completed by phagocytosis.7,8,9,18,20,29,31 In line with the general function of macroautophagy, namely being the major inducible pathway for degradation of cytoplasmic components including whole organelles, autophagic PCD predominantly appears to be activated when the developmental programme or in adulthood, homeostatic mechanisms demand massive cell elimination; in all cases, the bulk of cytoplasm is removed by autophagy before nuclear collapse ensues. In instances of cell injury, damaged organelles or membranes may be transferred into the autophagic pathway, serving as homeostatic mechanism at the subcellular scale, and that might be overwhelmed resulting in elimination of the whole cell. It is tempting to speculate that autophagic elimination of potentially harmful subcellular structures might be a functional analogue to the cell's safeguards controlling DNA repair and p53-mediated apoptotic suicide upon DNA damage. Finally, it should be noted that autophagic cell death and apoptosis are not mutually exclusive phenomena. Thus, both types of cell death can occur simultaneously in tissues,7,11,27,28,29,35,38 but also subsequently as governed by the developmental programme.31 Moreover, dying cells may share apoptotic and autophagic features (‘mixed type’).7,11,20,21,37,38,40,41,42,43,44 The determinants for the eventual manifestation of either type of programmed cell death are poorly understood.
Figure 1
Development and patterns of cell death. (1) Commonalities of apoptosis and autophagic cell death: see text. Apoptosis: (2) condensation of cytoplasm and of chromatin at the nuclear membrane to sharply delineated masses (often like crescents). (3) cell fragmentation into apoptotic bodies. (4) Phagocytosis (in vivo) and heterophagic degradation. Note: according to original description autophagy/lysosomes do not play a distinct role early in apoptosis. Autophagic cell death: (2) Autophagy: formation of autophagic vacuoles (AVs; open circles) and degradation of cytoplasmic constituents; (3) Pyknosis, single pyknotic mass in the centre of the nucleus, nuclear envelope still intact, cytoplasm amorphous with few clusters of AVs and mitochondria (as observed in MCF-7 cells). Note: autophagocytosis with apoptotic-like DNA condensation/fragmentation may also occur; (4) Phagocytosis (in vivo) and final degradation. (5) A cell may enter apoptosis or autophagic cell death which, however, may not be completed and secondary necrosis ensues. For references: see text
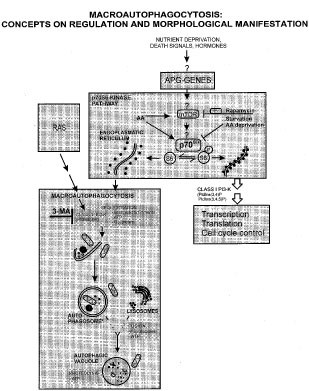
Figure 2
Macroautophagocytosis: concepts on regulation and morphological manifestation. Macroautophagy: For description see text. Note: simplified presentation as early and late autophagosomes, acidification, integration of autophagosomal membrane into that of autophagic vacuole etc. are not indicated (for review:39,45,46). Furthermore, cytoplasmic components may also be degraded by microautophagy, crinophagy, hsc73 chaperone-mediated autophagy, or non-lysosomal pathways including the ubiqutin-proteasome pathway and calpains as reviewed elsewhere.46,47,48 p70S6kinase pathway: Schematic diagram following Dennis et al.,61 (see text for explanation and references). APG genes: autophagic defective genes; mTOR: mammalian target of rapamycin. Rapamycin complexes with FKBP-12 (FK506 binding protein) to inhibit mTOR-phosphorylation. p70S6k: p70S6-kinase; hypophosphorylated 70S6k promotes detachment of ribosomes from RER and sequestration/autophagy; in contrast, hyperphosphorylated p70S6k promotes attachment of ribosomes to ER. →: Stimulation; —| inhibition
Autophagic PCD: from morphology to molecular events
Current concepts on macroautophagy suggest that it ensues through a sequence of morphological visible events which are highly conserved from yeast to humans (for review:39,45,46). Briefly, the macroautophagic pathway in mammalian cells starts with the sequestration of cytoplasmic material to form an early autophagosome (Figure 2). The double-membrane of the early autophagosomes is generally considered to derive from ribosome-free regions of the endoplasmic reticulum; alternatively, it has been suggested to originate from a related organelle named ‘phagophore’ or from post-Golgi membranes (for review:39,45,46). Autophagic vacuoles (autolysosomes) result from fusion of late autophagosomes with lysosomes; thereby, the final degradation of the sequestered cytoplasmic material is triggered (Figure 2). Cytoskeletal proteins are an integral part of this pathway; the sequestration requires intermediate filaments (cytokeratin and vimentin), the movement and fusion of lysosomes with the late autophagosomes requires the microtubular system (for review39,45,46). All steps including the final degradation of sequestered cytoplasmic material in autolysosomes are ATP-dependent (Figure 2; for review:39,45,46).
A functional link between macroautophagoytosis and cell suicide could be established by inhibition experiments with 3-methyladenine (3-MA).40,44,49,50,51,52,53,54 3-MA has been described to specifically block the sequestration step;49 at the molecular level 3-MA has been found to inhibit class III phosphatidylinositol-kinase activity (Figure 2).54 Thus, 3-MA has been found to inhibit both, the formation of autophagic vacuoles and the eventual cell death as indicated by nuclear destruction in a number of experimental settings including tamoxifen treated human mammary carcinoma cells (MCF-7), gastric and glioma cells overexpressing Ras, TNF-α treated human T-lymphoblastic leukaemic cells, neuronal cells upon serum withdrawal or arabinoside, kidney cells lines treated with bacterial toxins such as ricin, abrin, Shiga toxin and diphtheria toxin.40,44,49,50,51,52,53 In view of functional criteria for the differentiation between subtypes of PCD, it is of interest to mention preliminary data suggesting that 3-MA does not inhibit TGF-β1 induced apoptosis of hepatocytes (W Parzefall, personal communication).
Furthermore, possible interactions of mechanisms controlling the biogenesis of lysosomes55 and their subsequent fusion with autophagosomes with those of the cell's suicide have to be considered. Early studies on regressing endocrine-dependent tumours suggested the involvement of _de-novo_-synthesis and an increased activity of lysosomal enzymes.56 Both, the increment in number and activity of lysosomes were considered to be the effect, not the cause, of tumour regression.56 More recently, TNF-α was found to induce an autophagic type of cell death in T-lymphoblastic leukaemic cells; 3-MA inhibited both the formation of autophagosomes and cell death.50 However, asparagine, which inhibits the fusion of lysosomes with autophagosomes, did not prevent TNF-α induced cell death.50 Thus, inhibition of an event downstream of sequestration (Figure 2) did not affect the execution of autophagic cell death, suggesting that the supply of lysosomes might not be a check point for initiation of this type of programmed cell death. Likewise, tamoxifen-induced autophagic cell death in MCF-7 cells was not associated with an expansion of the lysosomal compartment as indicated by biochemical and histochemical means (Török and Bursch, unpublished observation). Furthermore, 3-MA is known to slightly increase lysosomal pH in hepatocytes.51 However, increase of the lysosomal pH by monensin or NH4Cl did not protect kidney cells against ricin-induced lysis, thus excluding a possible increase in lysosomal pH as cause for the protective action of 3-MA.51 A cautionary note on the role of lysosomal enzymes during cell death has been published recently.57 Beem et al.57 suggested that misconceptions may emerge because of preparatory artefacts resulting in breakage of apoptotic bodies during tissue homogenisation. It may well be that breakage of apoptotic bodies during homogenisation of tumours is the cause for an observed increase in soluble beta-glucuronidase activity, while the lysosomes of the ingesting tumour cells remain intact.57 In summary, at present the interactions of lysosome biogenesis with the pathway(s) leading to autophagic cell death remain elusive. However, the current data based on functional criteria suggest that the sequestration step might provide a superior regulatory link between autophagocytosis and cell suicide rather than downstream events in the autophagic pathway.
As to biochemical characteristics, recent evidence suggests that the cytoskeleton exhibited distinct fates during autophagic and apoptotic cell death. In apoptosis, the cell's preparatory as well as executional steps include depolymerisation or cleavage of actin, cytokeratins, lamins and other cytoskeletal proteins, most probably resulting in the typical final shape of apoptotic cells (details and references in2,58). In contrast, as exemplified by autophagic death of MCF-7 cells after tamoxifen, the cytoskeleton was found to be redistributed but largely preserved, even in cells exhibiting nuclear condensation/fragmentation (i.e. irreversible stage of cell death).58 A pronounced fragmentation of the cytokeratin could not be detected before MCF-7 cells detached from the substrate and therefore probably were in a stage of secondary necrosis (Figure 1).58 Remarkably, the vast majority (about 85%) of MCF-7 cells exhibiting a pyknotic nucleus still contained F-actin as demonstrated by its interaction with phalloidin.58 Polymerisation of G- to F-actin is an ATP-dependent process and therefore, F-actin is a sensitive indicator of the metabolic state of a cell. In support of this notion, electronmicroscopy and rhodamine 123 staining revealed that at late stages of the death process the cytoplasm appeared amorphous, but the few remaining autophagic vacuoles were associated with clusters of structurally and functionally intact mitochondria.44,58 The preservation of mitochondria and thus ATP synthesis throughout autophagic degradation was also supported by the observation that mitochondrial dehydrogenase-activity did not decrease with time in TAM treated MCF-7 cultures (Török and Bursch, unpublished observation). It appears likely, that ATP synthesis is maintained at a level required for the completion of autophagocytosis. Moreover, the protein cross-linking enzyme transglutaminase, which is activated in apoptotic hepatocytes,59 is not involved in tamoxifen induced PCD of MCF-7 cells. Thus, the preservation of the cytoskeleton during autophagic death of MCF-7 cells matches with current concepts on the cytoskeleton's function in macroautophagy and furthermore, strongly supports the morphological evidence that apoptosis and autophagic reflect distinct pathways of cell suicide.
The genetics and signalling of macroautophagy is best studied in yeast, but – like its morphological appearance – the molecular events of initiation and execution of macroautophagy have been found to be highly conserved from this organism to humans.39,46,60,61 Following Dennis et al.61 a hypothetical model on molecular control of autophagy based upon yeast and mammalian data is depicted in Figure 2 (‘p70S6-kinase pathway’). In yeast, to date 14 Apg-genes (autophagy-defective genes) are known to act in a conjugation cascade as reviewed elsewhere.60 Two mammalian homologues of the Apg gene family have been identified, namely ASP/hApg5 (ASP: apoptosis specific protein;62 and Apg6/vps30 (beclin-1).63,64 ASP/hApg5 was first described as ‘apoptosis specific protein’ because of its expression in Burkitt's lymphoma, transformed retinoblasts and a number of other human and rodent cell lines during apoptosis (references in62). DNA sequencing revealed this protein to be homologous the yeast Apg5 gene. To date however, a functional link between ASP/Apg5 expression and cell death has not been established. Most recently, beclin-1 was the first gene described to induce autophagocytosis in mammalian cells.63,64 Beclin 1 is a bcl-2-interacting protein with structural similarity to the yeast autophagy gene apg6/vps30. It was found to be expressed ubiquitously at high levels in normal breast epithelia, but mono-allelically deleted in 40–75% of sporadic human breast cancers and ovarian cancers. Beclin-1 promoted autophagy in yeast and in human MCF-7 breast carcinoma cells; beclin-1 induced formation of autophagic vacuoles was prevented by 3-MA. The autophagocytosis-promoting activity of beclin 1 in MCF-7 cells was associated with inhibition of MCF-7 cell proliferation, in vitro clonigenicity and tumorigenesis in nude mice. In these studies, no evidence for an enhanced rate of cell death in MCF-7.beclin1 clones was found using trypanblue exclusion as an indicator of cell viability.64 However, previous studies of our own with MCF-7 cells revealed that the manifestation autophagic cell death by nuclear condensation/fragmentation was neither associated with release of cytoplasmic enzymes into the culture medium nor with a significant trypanblue staining of dead cells, thus matching with the metabolic requirements of autophagocytosis.39,45 These observations suggest that autophagic cell death – like apoptosis – at least in early stages is not associated with loss of cell membrane integrity. Thus, whether beclin-1 also might induce cell death cannot be excluded yet. Downstream of Apg-genes act TOR (target of rapamycin) and p70S6-kinase; the TOR/p70S6-kinase pathway plays an important role in balancing anabolic and katabolic states of cells.45,61,65,66,67,68 Hypophosphorylated p70S6-kinase promotes detachment of ribosomes from endoplasmic reticulum; this is considered to be one of the initial molecular events in sequestration (for review:39,45,46). As outlined above, the sequestration step provides an important regulatory link between autophagocytosis and cell suicide and therefore, the TOR/p70S6-kinase pathway appears to be a promising target for studying the interaction between autophagocytosis and cell death.
Studies by Kuchino et al.52,53 on the RAS-signalling pathway provided the first clear evidence for molecular interactions between autophagy and programmed cell death in mammalian cells. It has been shown that the expression of oncogenically mutated ras gene in human glioma and gastric cancer cell lines induces cell death including autophagocytosis. The nuclei remained relatively well-preserved and were negative for TUNEL staining,52,53 thus matching with the general morphological features of type II PCD (cf Table 1). The oncogenic Ras-induced cell death was dependent on the activity of phosphatidylinositol-3-kinase (PI3-K), a physiological downstream effector of Ras.53 A seemingly paradox was that PI3-K activity is required for both, induction of autophagocytosis but also for S6-phosphorylation of the ribosomal protein S6 and consequently, block of autophagocytosis (see above). However, recent studies on human cancer cells revealed that distinct classes of phosphatidylinositol-3-kinases act in opposite directions in the pathways signalling for sequestration.54 Thus, the class III PI3-K product PtdIns(3)P is required for sequestration; formation of PtdIns(3)P as well as of autophagosomes was found to be inhibited by 3-MA, wortmannin and LY294002.46,54 On the other hand, increasing the class I PI3-K products PtdIns(3,4)P and PtdIns(3,4,5)P inhibits macropautophagocytosis and favours protein synthesis, cell proliferation and cell survival (Figure 2).46,54,67 Furthermore, Ras-induced cell death occurred in the absence of caspase activation, it did not require wt-p53 activity and was not inhibitable by the anti-apoptotic Bcl-2 protein.53 Notably, the functional effector machinery for the execution of apoptosis could be activated in the Ras-transformed cells by appropriate signals, demonstrating that the manifestation of autophagic cell death does not simply reflect defective apoptosis.53 Thus, these studies strongly suggest that autophagic cell death may be assigned to the caspase-independent type(s) of programmed cell death including apoptosis, as recently reviewed elsewhere.2,52,69,70 Likewise, studies on isolated neurons revealed that the manifestation of autophagic cell death may be controlled upstream of caspase cascades, but downstream of JNK/p38 (after NGF-withdrawal) and p53 (after cytosine arabinoside).40 These studies also suggested that the same apoptotic signals that target mitochondria also activate autophagy. Once activated, autophagy may mediate caspase-independent neuronal cell death.40
Taken together, although a few molecular features (apg-genes, Ras, PI3-K) may be assigned to the morphological manifestation of autophagic cell death, the mechanisms of initiation and execution of this type of programmed cell death are still enigmatic. However, the few tesseras on molecular interactions between autophagy and cell death obtained so far support the concept that both processes share common signalling pathways. The apg-gene family and TOR/p70S6 kinase pathway provide most likely candidates. The RAS–signalling in autophagic cell death, for instance might interact with this pathway by crosstalk via PI3-kinases. Furthermore, as outlined above apoptosis and autophagic cell death are not mutually exclusive phenomena, but may occur in the same cell. In support and extension of the morphological observations, recent high-throughput proteom analyses revealed evidence that autophagic death of tamoxifen treated MCF-7 cells and CD95-induced apoptosis in Jurkat cells shared some commonalities as exemplified by the cell's stress response (translocation of heat shock proteins).12,71
Differences and commonalities of autophagocytosis and phagocytosis
Autophagocytosis and phagocytosis share a dynamic reorganisation in the structure and composition of membranes.5,45,46,72 In case of phagocytosis, ligation of external particles such as apoptotic bodies to the phagocyte membrane initiates its reorganisation; the ligation appears to be driven by surface tags of apoptotic bodies or by release of soluble factors from dying cells targeted at receptors of the phagocytes.5,46,72,73 Engulfment may be considered functionally equivalent to the sequestration step during autophagocytosis; as initial events both precede (auto)phagosome formation. Biochemically, these steps share their requirement for actin.5,45,46,72 Notably, the size limits of particles for being processed through either pathway are in the same range, namely 300–900 nm in diameter.46,72,73 Like the tagging of apoptotic cells, the targeting of mitochondria, peroxisomes, ER-membranes and cytosolic constituents for sequestration appears closely regulated. Macroautophagy can operate with selectivity for certain subcellular structures over others, but also in a largely nonselective fashion (for review:39,45,46). For instance during regression of chemically induced rat liver hypertrophy, selective autophagic elimination of either smooth endoplasmic reticulum or peroxisomes (pexophagy) has been observed; mitotic chromosomes43 and damaged mitochondria can be eliminated by the same way (for review:39,45,46). In general, the underlying mechanisms for selection or exclusion of cell components for/from autophagy are poorly understood. Future studies will have to address the underlying molecular events and their link to cell death signalling pathways.
Remarkably, a number of observations suggested that the autophagic type of cell death ensues independent of caspases.52,69,70 In apoptosis, the caspase cascades provide a powerful tool to mediate diverse pro-apoptotic signals to a ‘final common pathway’; most if not all the prominent morphological features of apoptosis as originally described are caspase-dependent.2 From a teleological point of view, apoptosis is designed to delete cells from tissues rapidly; the clearance of apoptotic cell residues from tissues is facilitated by tagging them for phagocytosis5 as well as by volume reduction (condensation/fragmentation) being appropriate for phagocytosis (300–900 nm). What would be the advantage for activating an autophagic type of cell suicide? Hypothetically, self-digestion preceding suicide might reduce the functional load imposed on the surviving cells by phagocytosis and break down of huge amounts of dead cells as necessary in remodelling tissues; thereby, a rapid elimination of cells would be facilitated and would help to prevent inflammatory and immunological responses.5 In addition, soluable molecules resulting from autophagic breakdown might be recycled by other mechanisms such as pinocytosis.
The final (post mortem) degradation of apoptotic bodies as compared to the final stages of autophagocytosis deserves a comment. For instance, in the liver in vivo the morphological (incl. size) and histochemical features of phagocytosed apoptotic bodies closely resemble those of autophagic vacuoles.74,75 Thus, the final lysis and reutilisation of the digested material seems very likely not to differ significantly except its duration: in the liver in vivo, the half-life time for the clearance of apoptotic bodies was found to be about 120 min,75 that of autophagic vacuoles ranged from 5–45 min, depending on the material subjected for degradation.76 From a practical point of view, the similarities in the post mortem appearance of apoptotic bodies and autophagic vacuoles are of importance as the unequivocal identification and quantitative analysis of either phenomenon can be affected. For instance, in in vivo studies on small intestinal crypts of normal mice and Crocker mouse ascites tumours treated with cytostatic drugs, phagocytosed apoptotic bodies have been mistaken for autophagic vacuoles.77 However, apoptotic bodies (ABs) can be discriminated from autophagic vacuoles (AVs) based upon the chromatin residues present in ABs, but usually not in AVs; electronmicroscopy and/or specific stains to visualise DNA revealed to be most helpful tools as reviewed previously.75 Finally, in cultured cells cytoplasmic vacuolisation is widely observed, but this type of vacuolisation is considered to be distinct from that consequently to autophagy (for review:78). Taken together, these phenomena should be taken into account and appropriate techniques should be used to verify gross morphological observations.
Lysosomes in cell death
Early after discovery, lysosomes (‘lytic bodies’) have been associated with necrosis ensuing after cell damage, but are not generally considered as its primary cause.79,80,81,82 For instance, a close time course study on chemical hypoxia induced cell damage in cultured hepatocytes, namely ATP-deletion, bleb formation with cellular swelling, onset of a mitochondrial permeability transition, disintegration of lysosomes, plasma membrane failure from bleb rupture, and cell death has been published more recently.82 This study suggests that the release of hydrolytic enzymes from lysosomes may be the final event causing lysis of the membrane and irreversible loss of viability.82
However, activation of lysosomal enzymes is not restricted to the necrotic type of cell death. Thus, a number of lysosomotropic agents has been described to induce apoptosis (Figure 3).83,84,85,86,87,88,89,90 The potency of lysosomal enzymes to trigger apoptosis revealed these organelles as potential targets for increasing a cell's sensitivity for photodynamic therapy, for instance by facilitating oxidative stress via intralysosomal fenton-like reactions.83,91,92,93 In general, the magnitude of lysosomal rupture and consequently, the amount of hydrolytic enzymes released into the cytosol may induce either reparable sublethal damage, apoptosis, or necrosis;86,88,94,95 the dose-dependency of causing either apoptosis or necrosis is exemplified by the lysosomotropic agent MSDH (apoptosis ⩽50 μM vs necrosis ⩾75 μM; Figure 3).88 The decision between necrosis or apoptosis may also depend upon the organelle being targeted primarily as shown in murine leukaemia L1210 cells treated with the photosensitising agent chlorin e6 triacetoxymethyl ester: a low dose targeted mitochondria and triggered apoptosis, whereas a higher dose targeted lysosomal membranes with cell death likely occurring via a necrotic process.95
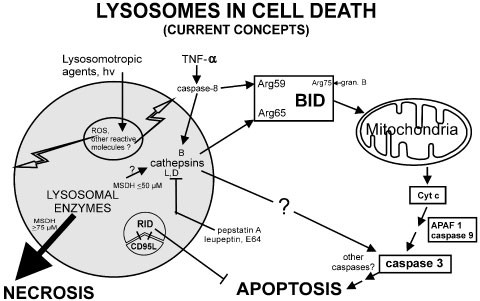
Figure 3
Potential roles of lysosomes in cell death. Summary of current concepts on the role of lysosomes for the induction of cell death, see text for explanation and references. Note: simplified as not distinguished between different stages of lysosome maturation. Lysosomotropic agents: α-tocopheryl succinate84; 9-Acetoxy-2,7,12,17-tetrakis-(beta-methoxyethyl)-porphycene (ATMPn)87; O-methyl-serine dodecyl-amide hydrochloride (MSDH)88; 5,8-dihydroxy-1,4-naphthoquinone89; chlorin e6 triacetoxymethylester (CAME)95; polyamine oxidase inhibitor MDL-72,527118; imidazo-acridinone C1311119; retinol120; Necrosis: 86,87,88,94,95
The lysosomal cystein proteases, cathepsins, have been implicated in the activation of caspases and apoptosis (Figure 389,90,96,97). For instance, studies on cultured fibroblasts and cardiomyocytes revealed that lysosomal destabilization (measured as release of cathepsin D) precedes release of Cytochrome c, loss of mitochondrial membrane potential and morphologic manifestation of apoptosis.89,90 Pepstatin A, an inhibitor of cathepsin D, was found to inhibit caspase-3-like proteolytic activity and to prevent apoptosis in several experimental settings.89,90,98,99 Notably, two p53 DNA-binding sites located in the cathepsin D-promoter have been found to specifically bind to p53 protein in vitro and appeared to mediate transactivation during p53-dependent apoptosis.99 Moreover, high levels of cathepsin D antisense RNA protected HeLa cells from interferon-gamma and Fas/APO-1-induced death.100 In transgenic models overexpression of cathepsin D induced or sensitised HeLa and PC12 cells to apoptosis upon serum deprivation.101 Other lysosomal cysteine proteases such as cathepsin B,C,L have been implicated in caspase activation as well.97,102,103 In particular, in cell free systems purified cathepsin B has been found to directly cleave caspase zymogens: it readily cleaved procaspase-11 and 1; procaspases 2, 6, 7 and 14 revealed to be weak, procaspase 3 a very poor and finally, procaspase 12 to be no substrate for cathepsin B.102 However, the physiological relevance of a direct physico-chemical interaction between cathepsin B and caspases has been challenged. Thus, Salvesen et al.104 most recently found no evidence for a direct role of lysosomal proteases in caspase activation. Rather, proteases that have leaked from lysosomes appear to cleave BID at Arg65 and thus, caspase activation may ensue via the mitochondrial pathway (Figure 3). The authors proposed that BID acts as a general sensor of proteolysis by endopeptidases and that this pathway enables cells to respond to adventitious and potentially harmful proteolysis by executing the apoptotic suicide.104 Likewise, cathepsin B has been found to contribute to TNF-α-mediated hepatocyte apoptosis by promoting mitochondrial release of Cytochrome c (Figure 3).105 The lysosomal cystein proteases, however, seem not to act exclusively via the mitochondrial Cytochrome c/APAF-1/caspase-9 cascade as evidence for a lysosomal-mediated activation of caspase-3 by a distinct pathway has been provided (Figure 3).106 Taken together, the specific roles of lysosomal cysteine proteases in the activation of caspases await elucidation. It should be also noted, that inhibition of cathepsins in neuronal cells and in primary hepatocyte cultures has been found to result in induction rather than inhibition of apoptosis.107,108 Finally, the plethora of lysosomal enzymes include nucleases and indeed, lysosomal endonucleases have been described giving raise for DNA fragmentation considered typical of apoptosis, using photo-oxidative damage to destabilise lysosomal membranes.85,86
Lysosomes have also been found to be involved in the control of CD95L presentation at the cell surface and thereby, of apoptosis. Thus, newly synthesised CD95L is stored in specialised secretory lysosomes in CD4+ and CD8+ T cells as well as natural killer cells; polarised degranulation controls the delivery of CD95L to the cell surface and eventually apoptosis.109 Likewise, in adenovirus infected cells the adenovirus RID (receptor internalisation and degradation) protein complex, mediates internalisation of cell-surface CD95 and its destruction inside lysosomes (Figure 3). Removal of CD95 from the surface of adenovirus-infected cells expressing RID may allow infected cells to resist CD95-mediated cell death and thus promote their survival.110 In vivo, promotion of cell survival has been observed in transgenic mice: congenital deficiency of lysosomal beta-glucuronidase results in prolongation of CrmA expression and thereby, inhibition of apoptosis.111 On the other hand, it should be reminded that in many cell types lysosomes secrete their content after fusion with the plasma membrane.112 Thus, secretory lysosomes of cytotoxic lymphocytes contain essential apoptotic molecules to eliminate virus-infected cells, namely the membranolytic perforin, and the serine protease granzyme B; the eventual cell death induced by granzyme B was found to be caspase-independent.113 Recently, dipeptidyl peptidase I, a lysosomal cysteine protease, has been found to be essential in the in vivo processing and activation of granzymes A and B.114
In conclusion, for many years lysosomal enzymes have been known to be involved (1) in necrotic type of cell lysis and, (2) in digestion of apoptotic cell residues upon their phagocytosis by vital neighbours, conceivably involving the whole plethora of lysosomal enzymes. More recently, accumulating evidence strongly suggests that lysosomal cysteine proteases may trigger preparatory steps of cell suicide; the underlying molecular mechanisms, however, are not yet elucidated. Nevertheless, diverse or relatively unspecific signals such as photodamage or lysosomotropic agents may be mediated to the specific enzyme cascades leading to coordinated final self-destruction of cells. The apparent role of lysosomes in programmed cell death adds support to the view that lysosomes are not simply a ‘garbage-disposal-unit’ as outlined recently by Luzio et al.55 Furthermore, the role of lysosomal system in programmed cell death deserves attention in view of their role in senescence and storage diseases.115
Conclusions
Programmed cell death (PCD) is an essential phenomenon in normal development and adulthood of multicellular organisms. Cells use different ways for active self-destruction, with the morphology ranging from apoptosis to autophagic cell death. Autophagic cell death appears to be activated when massive removal of cells or cytoplasm is demanded, for instance by developmental programmes. Autophagy preceeding cell death may also reflect a cell's adaptive response to sublethal (non-necrotic) conditions such as nutrient/growth factor deprivation or cell damage by cytotoxic drugs, hypoxia etc. A functional link is provided by a number of studies showing that 3-methyladenine inhibits both, formation of autophagosomes and the manifestation of cell death (nuclear collapse). However, so far no causative relationship between autophagocytosis and eventual cell death has been established. Nevertheless, some molecular features such as Ras-signalling, PI3-kinases and the autophagocytosis genes apg5/ASP and apg6/vps30 (beclin-1) might be assigned to pathways leading to the morphological appearance of autophagic cell death and provide promising targets for further studies. Furthermore, apoptosis and autophagic cell death are not mutually exclusive phenomena, they may occur simultaneously in tissues or even, conjointly in the same cell; both processes may end, if cell residues are not phagocytosed, in secondary necrosis. It should be emphasized, that programmed cell death appears to be highly conserved during evolution as it occurs in unicellular organisms,116 in the green algae Volvox spec regulating the germ-soma dichotomy,117 the slime mold Dicytostelium discoideum15,16 and, last but not least, in plants (see a series of reviews published in Cell Death and Differentiation 4(8), 1997). Golstein and coworkers raised the hypothesis that a single-core mechanism of PCD that may have emerged before the postulated multiple emergences of multicellularity has been raised.15,16 According to this hypothesis, the phenotypic variations of PCD would result from differences in enzymatic equipment and mechanical constraints adjusted to the given biological conditions.
Furthermore, there is sufficient evidence to suggest that lysosomes are important mediators of programmed cell death. Proteases released from the lysosomal compartment may trigger initiating events of apoptosis. Lysosomes may also be rate-limiting for the delivery of death receptors to the cell surface, thereby modulating the sensitivity of cells to external ligands. Taken together, these observations strongly suggest that lysosomes, like the mitochondria and the endoplasmatic reticulum, may play an important part in apoptosis signalling.
Abbreviations
Apg-genes:
autophagy-defective genes
ASP:
apoptosis specific protein
PCD:
programmed cell death
PI3-K:
phosphatidylinositol-3-kinase
MSDH:
O-methyl-serine dodecyl-amide hydrochloride
RID:
receptor internalization and degradation
ROS:
reactive oxygen species
TAM:
tamoxifen
TGF-β1:
transforming growth factor-β1
TNF-α:
tumor necrosis factor-α
TOR:
target of rapamycin
3-MA:
3-methyladenine
References
- Lockshin RA, Osborne B, Zakeri Z . 2000 Cell death in the third millennium Cell Death Differ. 7: 2–7
CAS PubMed Google Scholar - Hengartner MO . 2000 The biochemistry of apoptosis Nature 407: 770–776
Article CAS PubMed Google Scholar - Yuan J, Yankner BA . 2000 Apoptosis in the nervous system Nature 407: 802–809
CAS PubMed Google Scholar - Krammer PH . 2000 CD95’s deadly mission in the immune system Nature 407: 789–795
CAS PubMed Google Scholar - Savill J, Fadok V . 2000 Corpse clearance defines the meaning of cell death Nature 407: 784–788
CAS PubMed Google Scholar - Nicholson DW . 2000 From bench to clinic with apoptosis-based therapeutic agents Nature 407: 810–816
CAS PubMed Google Scholar - Beaulaton J, Lockshin RA . 1982 The relation of programmed cell death to development and reproduction: comparative studies and an attempt at classification Int. Rev. Cytol. 79: 215–235
CAS PubMed Google Scholar - Schweichel JU, Merker HJ . 1973 The morphology of various types of cell death in prenatal tissues Teratology 7: 253–266
CAS PubMed Google Scholar - Clarke PGH . 1990 Developmental cell death: morphological diversity and multiple mechanisms Anat. Embryol. 181: 195–213
CAS Google Scholar - Schwartz LM, Schmith SW, Jones MEE, Osborne B . 1993 Do all programmed cell deaths occur via apoptosis? Proc. Natl. Acad. Sci. USA 90: 980–984
CAS PubMed PubMed Central Google Scholar - Zakeri Z, Bursch W, Tenniswood M, Lockshin RA . 1995 Cell death: programmed apoptosis, necrosis, or other? Cell Death Differ. 2: 83–92
Google Scholar - Bursch W, Ellinger A, Gerner Ch, Fröhwein U, Schulte-Hermann R . 2000 Programmed cell death (PCD): apoptosis, autophagic PCD or others? Ann. N.Y. Acad. Sci. 926: 1–12
CAS PubMed Google Scholar - Kerr JFR, Wyllie AH, Currie AR . 1972 Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics Brit. J. Cancer 26: 239–257
CAS PubMed PubMed Central Google Scholar - Wyllie AH, Kerr JFR, Currie AR . 1980 Cell death: the significance of apoptosis Internat. Rev. Cytol. 68: 251–300
CAS Google Scholar - Cornillon S, Foa C, Davoust J, Buonavista N, Gross JD, Golstein P . 1994 Programmed cell death in Dictyostelium J. Cell Sci. 10: 2691–2704
Google Scholar - Olie RA, Durrieu F, Cornillon S, Loughran G, Gross J, Earnshaw WC, Golstein P . 1998 Apparent caspase independence of programmed cell death in Dictyostelium Curr. Biol. 8: 955–958
CAS PubMed Google Scholar - Hall D, Gu G, Garcia-Anoveras J, Gong L, Chalife M, Driscoll M . 1997 Neurology of degenerative cell death in Caenorhabditis elegans J. Neurosci. 17: 1033–1045
CAS PubMed PubMed Central Google Scholar - Beaulaton J, Lockshin RA . 1977 Ultrastructural study of the normal degeneration of the intersegmental muscles of Anthereae polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference of cellular autophagy J. Morphol. 154: 39–57
CAS PubMed Google Scholar - Jochova J, Quaglino D, Zakeri Z, Woo K, Sikorska M, Weaver V, Lockshin RA . 1997 Protein synthesis, DNA degradation, and morphological changes during programmed cell death in labial glands of Manduca sexta Dev. Genet. 21: 249–257
CAS PubMed Google Scholar - Dai JD, Gilbert LI . 1997 Programmed cell death of the prothoracic glands of Manduca sexta during pupal-adult metamorphosis Insect. Biochem. Mol. Biol. 27: 69–78
CAS PubMed Google Scholar - Dai JD, Gilbert LI . 1999 An in vitro analysis of ecdysteroid-elicited cell death in the prothoracic gland of Manduca Sexta Cell Tissue Res. 297: 319–327
CAS PubMed Google Scholar - Bowen ID, Mullarkey K, Morgan SM . 1996 Programmed cell death during metamorphosis in the blow-fly Calliphora vomitoria Microsc. Res. Tech. 34: 202–217
CAS PubMed Google Scholar - Jones HE, Bowen ID . 1993 Acid phosphatase activity in the larval salivary glands of developing Drosophila melanogaster Cell Biol. Int. 17: 305–315
CAS PubMed Google Scholar - Nardi JB, Godfrey GL, Bergstrom RA . 1991 Programmed cell death in the wing of Orgyia leucostigma (Lepidoptera: Lymantriidae) J. Morphol. 209: 121–131
CAS PubMed Google Scholar - Hinchliffe JR . 1981 Cell death in embryogenesis. In Cell death in biology and pathology, Bowen ID and Lockshin RA, eds London, New York: Chapman and Hall pp. 35–78
Google Scholar - Djechiche B, Segalen J, Chambon Y . 1994 Ultrastructure of mullerian and wolffian ducts of fetal rabbit in vivo and in organ culture Tiss. And Cell 26: 323–332
Google Scholar - Kaneko H, Ogiuchi H, Shimono -M . 1997 Cell death during tooth eruption in the rat: surrounding tissues of the crown Anat. Embryol. Berl. 195: 427–434
CAS PubMed Google Scholar - D'Herde K, De Prest B, Roels F . 1996 Subtypes of active cell death in the granulosa of ovarian atretic follicles in the quail (Coturnix coturnix japonica) Reprod. Nutr. Dev. 36: 175–189
CAS PubMed Google Scholar - Thorball N, Moe H, Winther-Nielsen H . 1985 Progressive involution and physiological death of smooth muscle cells of rat incisal arterioles Blood Vessels 22: 157–169
CAS PubMed Google Scholar - Shibahara T, Sato N, Waguri S, Iwanaga T, Nakahara A, Fukutomi H, Uchiyama Y . 1995 The fate of effete epithelial cells at the villus tips of the human small intestine Arch. Histol. Cytol. 58: 205–219
CAS PubMed Google Scholar - Verma V . 1983 Ultrastructural changes in human endometrium at different phases of the menstrual cycle and their functional significance Gynecol. Obstet. Invest. 15: 193–212
CAS PubMed Google Scholar - Migheli A, Piva R, Wei J, Attanasio A, Casolino S, Hodes ME, Dlouhy SR, Bayer SA, Ghetti B . 1997 Diverse cell death pathways result from a single missense mutation in weaver mouse Am. J. Pathol. 151: 1629–1638
CAS PubMed PubMed Central Google Scholar - Cataldo AM, Barnett JL, Berman SA, Li J, Quarless S, Bursztajn S, Lippa C, Nixon RA . 1995 Gene expression and cellular content of cathepsin D in Alzheimer's Disease brain: Evidence for early up-regulation of the endosomal-lysosomal system Neuron 14: 671–680
CAS PubMed Google Scholar - Cataldo AM, Ha, Milton DJ, Nixon RA . 1994 Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease Brain Res. 640: 68–80
CAS PubMed Google Scholar - Anglade P, Vyas S, Javoy F, Herrero MT, Michel PP, Marquez J, Mouatt Prigent A, Ruberg M, Hirsch EC, Agid Y . 1997 Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease Histol. Histopathol. 12: 25–31
CAS PubMed Google Scholar - Yamamoto S, Sawada K, Shimomura H, Kawamura K, James TN . 2000 On the nature of cell death during remodeling of hypertrophied human myocardium J. Mol. Cell Cardiol. 32: 161–175
CAS PubMed Google Scholar - Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, Kominami E, Uchiyama Y . 1995 Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis J. Neurosci. 15: 1001–1011
CAS PubMed PubMed Central Google Scholar - Rez G, Palfia Z, Fellinger E . 1991 Occurrence and inhibition by cycloheximide of apoptosis in vinblastine-treated murine pancreas. A role for autophagy? Acta Biol. Hung. 42: 133–140
CAS PubMed Google Scholar - Klionsky DJ, Emr SD . 2000 Autophagy as a regulated pathway of cellular degradation Science 290: 1717–1721
CAS PubMed PubMed Central Google Scholar - Xue L, Fletcher GC, Tolkovsky AM . 1999 Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution Mol. Cell Neurosci. 14: 180–198
CAS PubMed Google Scholar - Anderson KM, Seed T, Meng J, Ou D, Alrefai WA, Harris JE . 1998 Five-lipoxygenase inhibitors reduce Panc-1 survival: the mode of cell death and synergism of MK886 with gamma linolenic acid Anticancer res. 18: 791–800
CAS PubMed Google Scholar - Iwagaki H, Marutaka M, Mizukawa K, Kooka H, Tanaka N, Orita K . 1996 Changes in cellular ultrastructure induced by gamma-interferon in K562 cells may be prerequisite for apoptosis Acta. Med. Okayama. 50: 223–225
CAS PubMed Google Scholar - Qi L, Sit KH . 1998 Euchromatin megabase cleavages and conjoint apoptotic-autophagic death expression with nucleolar ball-and-socket joint dislocations in human Chang liver cells arrested in S-phase by etoposide Eur. J. Cell Biol. 77: 239–246
CAS PubMed Google Scholar - Bursch W, Kienzl H, Ellinger A, Török L, Walker R, Sikorska M, Pandey S, Schulte-Hermann R . 1996 Active cell death induced by antiestrogens tamoxifen and ICI 164384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy Carcinogenesis 17: 1595–1607
CAS PubMed Google Scholar - Blommaart EFC, Luiken JJFP, Meijer AJ . 1997 Autophagic proteolysis: control and specificity Histochem J. 29: 365–385
CAS PubMed Google Scholar - Kim J, Klionsky DJ . 2000 Autophagy, Cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells Annu. Rev. Biochem. 69: 303–342
CAS PubMed Google Scholar - DeMartino G and Slaughter C . 1999 The proteasome, a novel protease regulated by multiple mechanisms J. Biol. Chem. 274: 22123–22126
CAS PubMed Google Scholar - Carafoli E, Molinari M . 1998 Calpain-a protease in search and function Biochem. Biophys. Res. Comm. 247: 193–203
CAS PubMed Google Scholar - Seglen PO, Jordan PB . 1982 3-Methyladenine, a specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes Proc. Natl. Acad. Sci. USA 79: 1889–1892
CAS PubMed PubMed Central Google Scholar - Jia L, Dourmashkin RR, Allen PD, Gray AB, Newland AC, Kelsey SM . 1997 Inhibition of autophagy abrogates tumour necrosis factor alpha induced apoptosis in human T-lymphoblastic leukaemic cells Br. J. Haematol. 98: 673–685
CAS PubMed Google Scholar - Sandvig K, van Deurs B . 1992 Toxin-induced cell lysis: protection by 3-methyladenine and cycloheximide Exp. Cell Res. 200: 253–262
CAS PubMed Google Scholar - Kitanaka C, Kuchino Y . 1999 Caspase-independent programmed cell death with necrotic morphology Cell Death Differ. 6: 508–515
CAS PubMed Google Scholar - Chi S, Kitanaka C, Noguchi K, Mochizuki T, Nagashima Y, Shirouzu M, Fujita H, Yoshida M, Chen W, Asai A, Himeno M, Yokoyama S, Kuchino Y . 1999 Oncogenic Ras triggers cell suicide through the activation of a caspase-independent cell death program in human cancer cells Oncogene 18: 2281–2290
CAS PubMed Google Scholar - Petiot A, Ogier-Denis E, Blommaart EFC, Meijer AJ, Codogno P . 2000 Distinct classes of phosphatidylinositol 3′-kinases are involved in signalling pathways that control macroautophagy in HT-29 cells J. Biol. Chem. 275: 992–998
CAS PubMed Google Scholar - Luzio JP, Rous BA, Bright NA, Pryor PR, Mullock BM, Pier RC . 2000 Lysosome-endosome fusion and lysosomes biogeneses J. Cell Sci. 113: 1515–1524
CAS PubMed Google Scholar - Gullino PM . 1980 The regression process in hormone-dependent mammary carcinomas. In Hormones and Cancer, Iacobelli S, King RB, Lindner R and Lippman ME, eds New York: Raven Press pp. 271–279
- Beem EP, Hillebrand MJ, Benckhuijsen C, Overdijk B . 1997 Origin of the increased activity of beta-glucuronidase in the soluble fraction of rat mammary tumors during ovariectomy-induced regression Cancer Res. 198747: 3980–3987
Google Scholar - Bursch W, Hochegger K, Török L, Marian B, Ellinger A, Schulte Hermann R . 2000 Different fates of cytoskeletal filaments during autophagic and apoptotic types of programmed cell death in mammary carcinoma cells (MCF-7) and colon carcinoma cells (HT29HI1) J. Cell Science 113: 1189–1198
CAS PubMed Google Scholar - Bursch W, Fesus L, Schulte-Hermann R . 1992 Apoptosis (“programmed cell death”) and its relevance in liver injury and carcinogenesis. In Tissue Specific Toxicology, Dekant W and Neumann HG eds London: Academic Press pp. 95–117
- Mizushima N, Noda T, Yoshimori T, Ishii T, George MD, Klionsky Dj, Ohsumi M, Oshumi YA . 1998 Protein conjugation system essential for autophagy Nature 395: 395–398
CAS PubMed Google Scholar - Dennis PB, Fumagalli S, Thomas G . 1999 Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation Curr. Opinion Gen. Dev. 9: 49–54
CAS Google Scholar - Hammond EF, Brunet L, Johnson GD, Parkhill J, Milner AE, Brady G, Gregory CD, Grand RJA . 1998 Homology between a human apoptosis specific protein and the product of APG5, a gene involved in autophagy in yeast FEBS Lett. 425: 391–395
CAS PubMed Google Scholar - Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B . 1999 Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21 Genomics 59: 59–65
CAS PubMed Google Scholar - Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B . 1999 Induction of autophagy and inhibition of tumorigenesis by beclin 1 Nature 402: 672–676
CAS PubMed Google Scholar - Allison AC . 2000 Immunosuppressive drugs: the first 50 years and a glance forward Immunopharmacology 47: 63–83
CAS PubMed Google Scholar - Bonatti S, Pigullo S, Simili M, Abbondandolo A . 2000 Induction of apoptosis and inhibition of signalling pathways by alkylated purines Mutagenesis 15: 361–366
CAS PubMed Google Scholar - Shigemitsu K, Tsujishita Y, Hara K, Nanahoshi M, Avruch J, Yonezawa K . 1999 Regulation of translational effectors by amino acid and mammalian target of rapamycin signalling pathways. Possible involvement of autophagy in cultured hepatoma cells J. Biol. Chem. 274: 1058–1065
CAS PubMed Google Scholar - Mejillano M, Yamamoto M, Rozelle AL, Sun HQ, Wand X, Yin HL . 2001 Regulation of apoptosis by phosphatidylinositol 4,5 bisphosphate inhibition of caspases, and caspase inactivation of phosphatidylinositol phosphate 5 kinase J. Biol. Chem. 276: 1865–1872
CAS PubMed Google Scholar - Borner Ch, Monney L . 1999 Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ. 6: 497–507
CAS PubMed Google Scholar - Quignon F, De Bels F, Koken M, Feunteun J, Ameisen JC, de Thé H . 1998 PML induces a novel caspase-independent death process Nat. Gen. 20: 259–265
CAS Google Scholar - Gerner C, Fröhwein U, Gotzmann J, Bayer E, Gelbmann D, Bursch W, Schulte Hermann R . 2000 The Fas-induced apoptosis analyzed by high-throughput proteome analysis J. Biol. Chem. 275: 39018–39026
CAS PubMed Google Scholar - Tjelle TE, Lovdal T, Berg T . 2000 Phagosome dynamics and function Bioessays 22: 255–263
CAS PubMed Google Scholar - Kwiatkowska K, Sobota A . 1999 Signalling pathways in phagocytosis BioEssays 21: 422–431
CAS PubMed Google Scholar - Bursch W, Taper HS, Lauer B, Schulte-Hermann R . 1985 Quantitative Histological and Histochemical Studies on the Occurrence and Stages of Apoptosis (Controlled Cell Death) during Regression of Rat Liver Hyperplasia Vir. Arch. Abtl. B. Zellpathologie 50: 153–166
CAS Google Scholar - Bursch W, Oberhammer F, Schulte-Hermann R . 1992 Cell death by apoptosis and its protective role against disease Trends in Pharmacol. Sciences 13: 245–251
CAS Google Scholar - Papadopoulos T, Pfeifer U . 1986 Regression of rat liver autophagic vacuoles by locally applied cycloheximide Lab. Invest. 54: 100–107
CAS PubMed Google Scholar - Searle J, Lawson TA, Abbott PJ, Harmon B, Kerr JF . 1975 An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells J. Pathol. 116: 129–138
CAS PubMed Google Scholar - Henics T, Wheatley DN . 1999 Cytoplasmic vacuolation, adaptation and cell death: A view on new perspectives and features Biology of the Cell 91: 485–498
CAS PubMed Google Scholar - deDuve Ch, Wattiaux R . 1996 Functions of lysosomes Ann. Rev. Physiol. 28: 435–402
Google Scholar - Bowers WE . 1998 Christian de Duve and the discovery of lysosomes and peroxisomes Trends Cell Biol. 8: 330–333
CAS PubMed Google Scholar - Trump BE, Berezesky IK, Chang SH, Phleps PC . 1997 The pathways of cell death. Oncosis, apoptosis, and necrosis Toxicol. Pathol. 25: 82–88
CAS PubMed Google Scholar - Zahrebelski G, Nieminen AL, al-Ghoul K, Qian T, Herman B, Lemasters JJ . 1995 Progression of subcellular changes during chemical hypoxia to cultured rat hepatocytes: a laser scanning confocal microscopic study Hepatology 21: 1361–1372
CAS PubMed Google Scholar - Kessel D, Luo Y, Mathieu P, Reiners Jr JJ . 2000 Determinants of the apoptotic response to lysosomal photodamage Photochem. Photobiol. 71: 196–200
CAS PubMed Google Scholar - Neuzil J, Svensson I, Weber T, Weber C, Brunk UT . 1999 alpha-tocopheryl succinate-induced apoptosis in Jurkat T cells involves caspase-3 activation, and both lysosomal and mitochondrial destabilisation FEBS Lett. 445: 295–300
CAS PubMed Google Scholar - Monney L, Olivier R, Otter I, Jansen B, Poirier GG, Borner C . 1998 Role of an acidic compartment in tumor-necrosis-factor-alpha-induced production of ceramide, activation of caspase-3 and apoptosis Eur. J. Biochem. 251: 295–303
CAS PubMed Google Scholar - Olejnicka BT, Dalen H, Brunk UT . 1999 Minute oxidative stress is sufficient to induce apoptotic death of NIT-1 insulinoma cells APMIS 107: 747–761
CAS PubMed Google Scholar - Fickweiler S, Abels C, Karrer S, Baumler W, Landthaler M, Hofstadter F, Szeimies RM . 1999 Photosensitization of human skin cell lines by ATMPn (9-acetoxy-2,7,12,17-tetrakis-(beta-methoxyethyl)-porphycene) in vitro: mechanism of action J. Photochem. Photobiol. B. 48: 27–35
CAS PubMed Google Scholar - Li W, Yuan X, Nordgren G, Dalen H, Dubowchik GM, Firestone RA, Brunk UT . 2000 Induction of cell death by the lysosomotropic detergent MSDH FEBS Lett. 470: 35–39
CAS PubMed Google Scholar - Roberg K, Johansson U, Öllinger K . 1999 Lysosomal release of cathepsin D precedes relocation of cytochrome c and loss of mitochondrial transmembrane potential during apoptosis by oxidative stress Free Radic. Biol. Med. 27: 1228–1237
CAS PubMed Google Scholar - Ollinger K . 2000 Inhibition of cathepsin D prevents free-radical-induced apoptosis in rat cardiomyocytes Arch. Biochem. Biophys. 373: 346–351
CAS PubMed Google Scholar - Nilsson E, Ghassemifar R, Brunk UT . 1997 Lysosomal heterogeneity between and within cells with respect to resistance against oxidative stress Histochem. J. 29: 857–865
CAS PubMed Google Scholar - Terman A, Abrahamsson N, Brunk UT . 1999 Ceroid/lipofuscin-loaded human fibroblasts show increased susceptibility to oxidative stress Exp. Gerontol. 34: 755–770
CAS PubMed Google Scholar - Li W, Yuan XM, Brunk UT . 1998 OxLDL-induced macrophage cytotoxicity is mediated by lysosomal rupture and modified by intralysosomal redox-active iron Free Radic. Res. 29: 389–398
PubMed Google Scholar - Brunk UT, Svensson I . 1999 Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak Redox. Rep. 4: 3–11
CAS PubMed Google Scholar - Kessel D, Poretz RD . 2000 Sites of photodamage induced by photodynamic therapy with a chlorin e6 triacetoxymethyl ester (CAME) Photochem. Photobiol. 71: 94–96
CAS PubMed Google Scholar - Levy-Strumpf N, Kimchi A . 1998 Death associated proteins (DAPs): from gene identification to the analysis of their apoptotic and tumor suppressive functions Oncogene 17: 3331–3340
PubMed Google Scholar - Turk B, Turk D, Turk V . 2000 Lysosomal cysteine proteases: more than scavengers Biochim. Biophys-Acta. 1477: 98–111
CAS PubMed Google Scholar - Kanamori S, Waguri S, Shibata M, Isahara K, Ohsawa Y, Konishi A, Kametaka S, Watanabe T, Ebisu S, Kominami E, Uchiyama Y . 1998 Overexpression of cation-dependent mannose 6-phosphate receptor prevents cell death induced by serum deprivation in PC12 cells Biochem. Biophys. Res. Commun. 251: 204–208
CAS PubMed Google Scholar - Wu GS, Saftig P, El-Deiry WS . 1998 Potential role for cathepsin D in p53-dependent tumor suppression and chemosensitivity Oncogene 16: 2177–2183
CAS PubMed Google Scholar - Deiss LP, Galinka H, Barassi H, Cohen O, Kimchi A . 1996 Cathepsin D proteases mediates programmed cell death induced by interferon-y, FAS/APO-1 and TNF-a EMBO J. 15: 3861–3870
CAS PubMed PubMed Central Google Scholar - Shibata M, Kanamori S, Isahara K, Ohsawa Y, Konishi A, Kametaka S, Watanabe T, Ebisu S, Ishido K, Kominami E, Uchiyama Y . 1998 Participation of cathepsins B and D in apoptosis of PC12 cells following serum deprivation Biochem. Biophys. Res. Commun. 251: 199–203
CAS PubMed Google Scholar - Vancompernolle K, Van Herreweghe F, Pynaert G, Van de Craen M, De Vos K, Totty N, Sterling A, Fiers W, Vandenabeele P, Grooten J . 1998 Atractyloside-induced release of cathepsin B, a protease with caspase-processing activity FEBS Lett. 438: 150–158
CAS PubMed Google Scholar - Ishisaka R, Utsumi T, Yabuki M, Kanno T, Furuno T, Inoue M, Utsumi K . 1998 Activation of caspase-3-like protease by digitonin-treated lysosomes FEBS Lett. 435: 233–236
CAS PubMed Google Scholar - Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SL, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Brömme D, Krajewski S, Reed JC, Yin XM, Turk V, Salvesen GS . 2001 Lysosomal protease pathways to apoptosis: cleavage of Bid, not pro-caspases, is the most likely route J. Biol. Chem. 276: 3149–3157
CAS PubMed Google Scholar - Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufman SH, Gores GJ . 2000 Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c J. Clin. Invest. 106: 1127–1137
CAS PubMed PubMed Central Google Scholar - Ishisaka R, Kanno T, Akiyama J, Yoshioka T, Utsumi K, Utsumi T . 2001 Activation of Caspase-3 by Lysosomal Cysteine Proteases and Its Role in 2,2′-Azobis-(2-Amidinopropane)Dihydrochloride (AAPH)-Induced Apoptosis in HL-60 Cells J. Biochem. 129: 35–41
CAS PubMed Google Scholar - Isahara K, Ohsawa Y, Kanamori S, Shibata M, Waguri S, Sato N, Gotow T, Watanabe T, Momoi T, Urase K, Kominami E, Uchiyama Y . 1999 Regulation of a novel pathway for cell death by lysosomal aspartic and cysteine proteinases Neuroscience 91: 233–249
CAS PubMed Google Scholar - Maeda S, Lin KH, Inagaki H, Saito T . 1996 Induction of apoptosis in primary culture of rat hepatocytes by protease inhibitors Biochem. Mol. Biol. Int. 39: 447–453
CAS PubMed Google Scholar - Bossi G, Griffiths GM . 1999 Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells Nat. Med. 5: 90–96
CAS PubMed Google Scholar - Tollefson AE, Hermiston TW, Lichtenstein DL, Colle CF, Tripp RA, Dimitrov T, Toth K, Wells CE, Doherty PC, Wold WS . 1998 Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells Nature 392: 726–730
CAS PubMed Google Scholar - Okuyama T, Kosuga M, Li XX . 1999 Gene therapy for acute hepatitis using CrmA gene transduction Hum. Cell 12: 125–130
CAS PubMed Google Scholar - Andrews NW . 2000 Regulated secretion of conventional lysosomes Trends Cell Biol. 10: 316–321
CAS PubMed Google Scholar - Trapani JA, Jans DA, Jans PJ, Smyth MJ, Browne KA, Sutton VR . 1998 Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent J. Biol. Chem. 273: 27934–27938
CAS PubMed Google Scholar - Pham CT, Ley TJ . 1999 Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo Proc. Natl. Acad. Sci. USA 96: 8627–8632
CAS PubMed PubMed Central Google Scholar - Cuervo AM, Dice LF . 2000 When lysosomes get old Exp. Gerontol. 35: 119–131
CAS PubMed Google Scholar - Ameisen JC . 1996 The Origin of Programmed Cell death Science 272: 1278–1279
CAS PubMed Google Scholar - Kirk DL, Baran GJ, Harper JF, Huskey RJ, Huson KS, Zagris N . 1987 Stage-specific hypermutability of the regA locus of Volvox, a gene regulating the germ-soma dichotomy Cell 48: 11–24
CAS PubMed Google Scholar - Dai H, Kramer DL, Yang C, Murti KG, Porter CW, Cleveland JL . 1999 The polyamine oxidase inhibitor MDL-72,527 selectively induces apoptosis of transformed hematopoietic cells through lysosomotropic effects Cancer Res. 59: 4944–4954
CAS PubMed Google Scholar - Burger AM, Jenkins TC, Double JA, Bibby MC . 1999 Cellular uptake, cytotoxicity and DNA-binding studies of the novel imidazoacridinone antineoplastic agent C1311 Br. J. Cancer 81: 367–375
CAS PubMed PubMed Central Google Scholar - Fröhlich E, Wahl R . 1999 Effects of retinol on follicular porcine thyrocytes in culture J. Mol. Med. 77: 189–192
PubMed Google Scholar
Acknowledgements
I would like to thank Rolf Schulte-Hermann for critical reading of the manuscript.
Author information
Authors and Affiliations
- Institut für Krebsforschung der Universität Wien, Borschkegasse 8a, Wien, A-1090, Austria
W Bursch
Authors
- W Bursch
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toW Bursch.
Additional information
Edited by M Piacentini
Rights and permissions
About this article
Cite this article
Bursch, W. The autophagosomal–lysosomal compartment in programmed cell death.Cell Death Differ 8, 569–581 (2001). https://doi.org/10.1038/sj.cdd.4400852
- Received: 03 October 2000
- Revised: 19 January 2001
- Accepted: 01 February 2001
- Published: 10 July 2001
- Issue Date: 01 June 2001
- DOI: https://doi.org/10.1038/sj.cdd.4400852