Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9 (original) (raw)
Introduction
The Caenorhabditis elegans cysteine protease, CED-3, and its mammalian homologues, constitute the effector arm of the apoptotic machinery. These enzymes are produced as zymogens which undergo processing into two subunits of ∼20 and ∼10 kDa that are assembled into the active enzyme (reviewed in references1,2). Mammalian cells have at least two distinct caspase activation pathways.3 Death receptors of the tumour necrosis factor receptor (TNFR) family recruit FADD, an adaptor molecule which in turn recruits pro-caspase-8 or -10 to the death inducing signalling complex (DISC).4,5 The recruitment of pro-caspase-8 or -10 to the DISC promotes caspase-8/-10 processing6,7 by a mechanism that is thought to involve proximity-induced autoactivation of the pro-caspases.8 The second apoptosis signalling pathway is regulated by the Bcl-2 family of proteins and is initiated by a different set of initiator caspases and their adaptors. Mammalian caspase-9 contains an N-terminal caspase recruitment domain (CARD), which mediates interaction with its adaptor Apaf-1.9 Once activated, Apaf-1 recruits pro-caspase-9 to form the apoptosome protein complex. In a manner analogous to pro-caspase-8 activation, recruitment of pro-caspase-9 via its adaptor serves to bring pro-caspase molecules into close proximity, precipitating their activation.9,10
Structurally, mammalian caspase-2 closely resembles caspase-9 and CED-311,12 and previous studies have implicated caspase-2 in a variety of cell death pathways.11,13 The question, however remains, at what stage in the apoptotic process does caspase-2 act, since it has features of both upstream caspases (CARD pro-domain) and effector caspases (DEXD substrate specificity).14 Caspase-2 CARD has been shown to interact with the CARD present in the adaptor protein RAIDD,15 which when overexpressed in mammalian cells induces apoptosis. It remains, however, unclear whether RAIDD is necessary for caspase-2 activation16 and whether it plays a role in the apoptotic process. In many cell types, caspase-2 is processed early during apoptosis.17 Furthermore, overexpressed procaspase-2 can homodimerise in a CARD dependent manner and autoprocess.18,19 However, other studies have indicated that caspase-2 functions further downstream as an effector caspase. For example, caspase-3 can efficiently process pro-caspase-2,20 indicating that caspase-2 activation may occur downstream of this effector caspase. One possible scenario is that initial activation of caspase-2 occurs by an autocatalytic mechanism, but once effector caspases, such as caspase-3, have been activated, they are responsible for the bulk of pro-caspase-2 processing, forming an amplification loop.
In order to address the issue of whether caspase-2 is an initiator or effector caspase, we generated highly specific monoclonal antibodies against this protease. Using thymocytes derived from Apaf-1−/− and caspase-9−/−-deficient mice, we demonstrate that at least in this cell type, caspase-9 and Apaf-1 are required for caspase-2 processing indicating that cleavage of caspase-2 lies downstream of the Apaf-1/caspase-9 apoptosome. Furthermore, we have generated caspase-2-deficient mice and show that caspase-2 is dispensible for apoptosis of thymocytes or DRG neuronal cells induced by cytokine withdrawal or several stress stimuli.
These antibodies allowed us to confirm and extend the expression pattern of caspase-2 in mouse tissues21 and an extensive array of cell lines and to determine its subcellular localisation by subcellular fractionation. We show that caspase-2 is expressed in most tissues and cell types and that a significant proportion of caspase-2 is in the nucleus and within the Golgi apparatus.
Results and discussion
Characterisation of monoclonal antibodies to Caspase-2
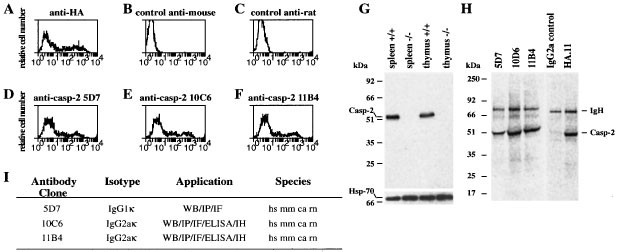
Monoclonal antibodies recognising native mouse caspase-2 were identified by immunofluorescence staining and flow cytometric analysis using a previously described protocol.22 293T cells transfected with a plasmid encoding a C-terminally hemagglutinin (HA)-tagged caspase-2 in which the active site cysteine was replaced by alanine were stained with hybridoma supernatants plus FITC-conjugated goat anti-rat IgG antibodies and analyzed in a FACScan. Antibodies specific to caspase-2 were revealed by a double immunofluorescence peak (Figure 1D–F) similar to the profile obtained by staining with the anti-HA epitope tag-specific antibody (Figure 1A). The peak with lower intensity represents background immunofluorescence of untransfected 293T cells and the higher intensity peak represents specific caspase-2 staining in the transfected HA-caspase-2 expressing 293T cells. From an initial screen of 2000 hybridoma cultures, 17 anti-caspase-2 antibody-secreting hybridomas were selected, expanded and subcloned.
Figure 1
Screening for caspase-2 specific monoclonal antibodies (mAbs). 293T cells were transiently transfected with a construct encoding a haemaglutinin (HA) epitope tagged caspase-2 mutant in which the active site cysteine had been replaced by alanine. Transfected cells were fixed, permeabilised and stained with mouse anti-HA mAb (A, positive control), with secondary antibody alone (B and C, negative controls), or the rat anti-caspase-2 mAbs 5D7 (D), 10C6 (E) or 11B4 (F). Staining was visualised by FITC-conjugated goat anti-mouse IgG antibodies (A, B) or FITC-conjugated goat anti-rat IgG antibodies (C–F). (G) Western blotting with anti-caspase-2 mAb 11B4 revealed caspase-2 protein in lysates from wild type (wt) spleens and thymi but not in tissues from caspase-2−/− mice (upper panel) (similar results were obtained with anti-caspase-2 clone 10C6) and probing with an anti-Hsp70 mAb was used as loading control (lower panel). (H) Immunoprecipitation with anti-caspase-2 mAbs 5D7, 10C6, 11B4, an isotype-matched control antibody or the anti-HA mAb of 35S-labelled 293T cells transfected with mutant caspase-2 HA. (I) Summary of the characteristics of the anti-caspase-2 mAbs 5D7, 10C6 and 11B4. All antibodies recognise human (hs), mouse (mm), monkey (ca) and rat (rn) caspase-2 protein by immunofluorescence staining (IF), immunopecipitation (IP), immunohistochemistry (IH), enzyme linked immunoadsorbant assay (ELISA) and Western blotting (WB)
Two independent mAbs, 10C6 and 11B4, that recognise mouse, human, rat and monkey caspase-2 in immunofluorescence staining, Western blotting and immunoprecipitation (Figure 1G–I), were chosen for further experiments. In tissues from normal mice both mAbs detected by Western blotting a single protein of ∼51 kDa, corresponding to endogenous full length pro-caspase-2 (Figure 1G). The 10C6 and 11B4 (Figure 1G) antibodies were highly specific for caspase-2 since no crossreactivity to other proteins was observed by Western blotting and no protein band was detected in tissues made from caspase-2−/− mice (Figures 1G, 2). These results document that the monoclonal antibodies 10C6 and 11B4 are suitable reagents to investigate caspase-2 expression, localisation and processing.
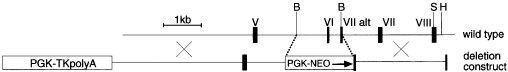
Figure 2
Targeted disruption of the mouse caspase-2 gene. Roman numerals denote exons. _Bam_HI, _Sal_I and _Hind_III sites are denoted ‘B’, ‘S’ and ‘H’, respectively. Exon VI which encodes for residues including the catalytic cysteine residue of caspase -2 and exon VII were all replaced by the PGK-NEO cassette
Caspase-2 activation during apoptosis
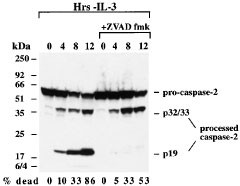
In cells undergoing apoptosis, both anti-caspase-2 mAbs detected not only the 51 kDa zymogen, but also of the products of caspase-2 processing, namely the 32/33 kDa doublet, and the 19 kDa subunit. These cleavage products were observed in BAF-3, FDC-P1 and HeLa cells upon apoptosis induction by a variety of stress conditions, such as IL-3 deprivation (Figure 3 and data not shown) or treatment with staurosporine or UV-irradiation (not shown). In all cases, processing of caspase-2 was rapid. For example, caspase-2 cleavage products became visible within 4 h of IL-3 withdrawal from BAF-3 cells. Interestingly, treatment of IL-3 deprived BAF-3 cells with the broad spectrum caspase inhibitor zVAD-fmk prevented the generation of p19 subunits, but had no impact on the appearance of the 32/33 kDa polypeptides, which probably represent prodomain-less caspase-2. As caspase-2 is insensitive to zVAD-fmk,23 this observation may indicate that the intermediate product in these cells is generated by autocatalytic activation of caspase-2, whereas, generation of the mature subunit, p19, may require a zVAD-fmk sensitive caspase, such as caspase-3. Alternatively, the first cleavage step may be mediated by an initiator caspase that is insensitive to zVAD-fmk or not easily accessible to zVAD-fmk perhaps due to sequestration inside an organelle.
Figure 3
Caspase-2 mAbs detect the products of caspase-2 processing during apoptosis induction. Cell death was induced in BAF-3 cells by withdrawal of IL-3 and cell lysates were prepared 0, 4, 8 and 12 h after apoptosis induction. Caspase-2 expression and processing was assessed by Western blotting using anti-caspase-2 mAb 11B4 and detected by ECL. Figures below the blot indicate the percentage of dead cells as assessed by trypan blue exclusion
Expression of caspase-2 in cell lines and mouse tissues
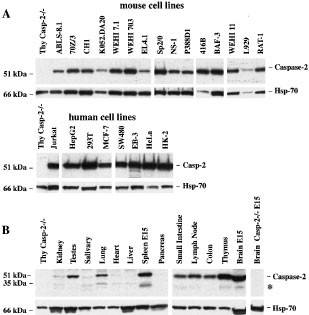
Caspase-2 expression in cell lines was determined by Western blotting. Examples of such Western blots are shown in Figure 4A and the overall results are summarised in Table 1. Readily detectable levels of caspase-2 were found in cell lines of lymphoid (B and T), myeloid, erythroid, fibroblast and epithelial origin. A broad survey of normal adult mouse tissues by Western blotting readily detected caspase-2 in the brain (E15), thymus, spleen, lymph nodes, colon, small intestine and testes (Figure 4B). Low levels of caspase-2 were found in the kidney, salivary gland and heart, but little or no caspase-2 expression could be detected in pancreas or liver (Figure 4B). This contrasts with the ubiquitous expression of caspase-2 mRNA observed during embryogenesis.11 This may indicate that caspase-2 expression differs between embryo and adult tissues. These results confirm previous observations on the tissue expression of caspase-221 and extend them by demonstrating that caspase-2 is also expressed at high levels in the testes and colon, is barely detectable in the salivary gland and absent from pancreas. Overall these results indicate a possible role for caspase-2 during development and in hematopoetic cells in the adult.
Figure 4
(A) Caspase-2 expression in cultured mouse and human cell lines. Caspase-2 protein was revealed in lysates from 2×105 mouse and human cell lines by Western blotting with anti-caspase-2 mAb 11B4 and detection by ECL. (B) Expression of caspase-2 by Western blot analysis in tissue lysates from normal and caspase-2−/− mice (250 μg total protein/lane). Probing with an anti-Hsp70 mAb was used as loading control for all blots. (*) may represent caspase-2 (short) or processed caspase-2
Table 1 Summary of Western blot analysis of caspase-2 protein expression in cell lines
The smaller ∼35 kDa band (*) observed in Western blots in some tissues, such as the lung, kidney, spleen (E15) and brain (E15) is most likely a processing intermediate (see above). It is, however, also possible that this ∼35 kDa band could be the putative short form of caspase-2 (Nedd2s/Ich-1s, 343 αα), which has been reported to be expressed at the mRNA level in similar tissues, such as the brain, skeletal muscle and to a lesser extent the spleen, lung, gut, testis and kidney.24 The function of Nedd2s/Ich-1s remains poorly defined, although it has been suggested to inhibit apoptosis in certain neuronal populations.21,25
Subcellular localisation of caspase-2
The subcellular localisation of pro-caspase-2 remains controversial. Some of us have previously shown that GFP-tagged pro-caspase-2 localises to the cytoplasm and the nucleus and can be processed in both compartments.19 Subsequent studies using subcellular fractionation and a polyclonal anti-caspase-2 antibody26 showed that pro-caspase-2 was located in the cytosol, nucleus and mitochondria.27 Recent studies by Mancini28 demonstrated that caspase-2 is predominantly located in the Golgi complex and nucleus.
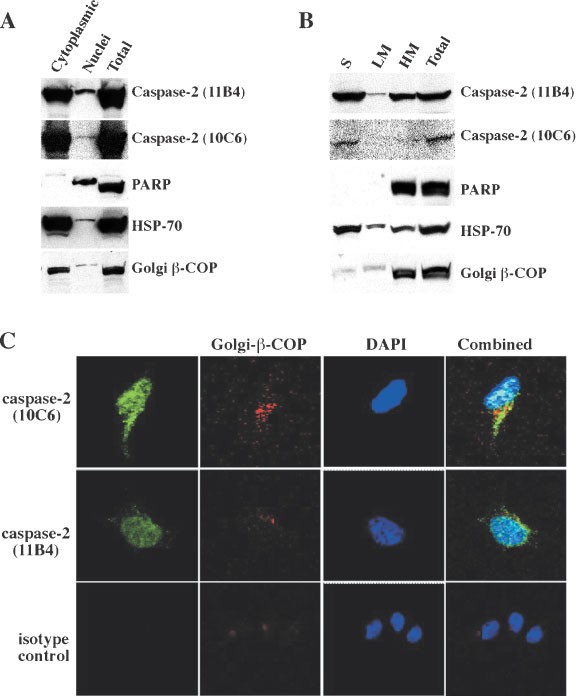
To determine the localisation of pro-caspase-2, we performed sub-cellular fractionation and confocal microscopy analyses on cells expressing endogenous caspase-2. We chose to mechanically disrupt cells by Dounce homogenisation, rather than lysing them with non-ionic detergents, because these reagents have been shown to cause artefactual changes in localisation of some cell death regulators.29 Subcellular fractions of healthy Jurkat cells were analyzed by immunoblotting with mAbs 10C6 and 11B4 (Figure 5A). Endogenous pro-caspase-2 protein was predominantly associated with the cytosolic fraction, the latter includes most of the Golgi apparatus (detected by anti-β-COP). A small but significant proportion of pro-caspase-2 protein was present in the nuclear fraction, which contained the nuclear protein PARP. Further fractionation of the cytoplasmic compartment of HeLa cells to obtain a light membrane (LM) and soluble fraction (S) revealed that caspase-2 was barely detectable in the LM fraction but present in the S fraction (Figure 5B). The presence of the large pro-caspase-2 pool in the soluble fraction may be due to weak attachment of pro-caspase-2 to intracellular membranes, which is easily disrupted during fractionation. Alternatively, some pro-caspase-2 might escape from nuclei during fractionation. To explore this further, we studied endogenous caspase-2 localisation by immunofluorescence staining and confocal microscopy in HeLa cells. Caspase-2 in these analyses was found in the nucleus since it co-localises with DAPI (Figure 5C) and in a cytoplasmic structure that corresponds to the Golgi apparatus since it co-localises with the anti-β-COP antibody (Figure 5C). Neither the fact that the cytosolic fraction contains most of the Golgi apparatus nor the immunofluoresence staining constitute definitive proof for the association of caspase-2 with this organelle, but they are highly suggestive.
Figure 5
Analysis of caspase-2 localisation by subcellular fractionation and confocal microscopy. (A) Jurkat cell lysates prepared by Dounce homogenisation were separated into nuclear and cytoplasmic fractions. Proteins in fractions were size-fractionated by SDS–PAGE, electroblotted onto membranes and immunoblotting performed with mAbs specific for the proteins indicated. Caspase-2 protein, although found in both the nuclear and cytoplasmic fractions was predominantly expressed in the cytoplasmic fractions in extracts from healthy cells. Data shown are representative of three independent experiments. (B) HeLa cell lysates were prepared by Dounce homogenisation and separated into heavy membrane (HM) and supernatant fractions, the supernatant was further fractionated into light membrane (LM) and soluble fractions (S). Proteins in fractions were size-fractionated by SDS–PAGE, electroblotted onto membranes and immunoblotting performed with anti-caspase-2 mAbs (10C6 or 11B4) or with mAbs specific for the proteins indicated. Caspase-2 is localised in the nucleus and Golgi apparatus in HeLa cells. (C) HeLa cells were fixed, permeabilised, stained with anti-caspase-2 mAbs (10C6 or 11B4) plus FITC-conjugated goat anti-rat IgG antibodies (green), rabbit anti-β-COP plus biotinylated goat anti-rabbit and streptavidin Texas Red (red) and DAPI (blue) and examined by confocal microscopy
Caspase-2 is not essential for apoptosis
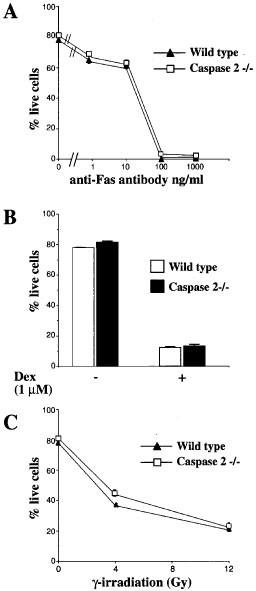
It has been speculated that in response to many stress signals, caspase-2 may act as either a positive or negative regulator of cell death. Decreasing caspase-2 levels by anti-sense technology was reported to delay cell death induced by growth factor deprivation in FDC-P1 cells30 and PC12 cells.13 Furthermore, caspase-2 deficient oocytes were shown to be resistant to apoptosis induced by chemotherapeutic drugs.21 However, oocytes may be unique in this regard, since, oocytes appeared to be the only cell types affected by lack of caspase-2,21 whereas caspase-2−/− lymphocytes were shown to be normally sensitive to chemotherapeutic drugs. We also generated caspase-2−/− mice (Figure 2) and found that in thymocytes, the absence of caspase-2 does not alter the kinetics of cell death or the dose response to a variety of cell death stimuli, including anti-Fas Ab (1-1000 ng/mL), the cytotoxic drug dexamethsone (1 μM) or γ-irradiation (4-12 Gy) (Figure 6A–C). This indicates that activation of caspase-2 is not essential for thymocyte apoptosis induced by these stimuli.
Figure 6
Capase-2 is dispensable for apoptosis in thymocytes. Cell death was induced in wt and caspase-2−/− thymocytes by treatment with 1–1000 ng/mL anti-Fas Ab (A), 1 μM dexamethasone (B) or γ-irradiation (4–12 Gy) (C). Cell viability was determined by staining with propidium iodide (PI) followed by FACS analysis. Error bars are S.E.M. from three replicate plates
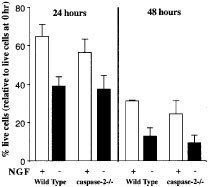
Dorsal root ganglion (DRG) neurons from postnatal day 2 wt or caspase-2−/− mice were cultured in the presence or absence of NGF and the percentages of live and dead cells at 24 and 48 h determined by using the MTT assay. DRG neurons from wt and caspase-2−/− mice died at the same rate (Figure 7). This contrasts with previous studies, which reported that decreasing caspase-2 levels by anti-sense technology delayed apoptosis induced by trophic factor deprivation in sympathetic neurons.13 Our observations are, however, in accordance with the observation that caspase-2 deficient sympathetic (SCG) neurons were normally sensitive to NGF withdrawal.21 Collectively, these studies indicate that apoptosis in thymocytes and neuronal cells can occur normally in the absence of caspase-2.
Figure 7
Caspase-2 is not required for trophic factor withdrawal induced apoptosis of DRG neurons. DRG neurons from postnatal day 2 (P2) caspase-2−/− mice or control (wt) C57BL/6 mice were cultured in the presence or absence of NGF. Cell viability was determined by the MTT assay and at each time point a minimum of five individual wells was counted. Data represent the mean of three separate experiments and at least four mice of each genotype were analyzed each time. The error bars represent±S.E.M.
Caspase-2 processing in mouse thymocytes requires Apaf-1 and caspase-9
Previous studies have indicated that pro-caspase-2 can be activated by autocatalysis in a proximity-induced manner.18,19 However, caspase-2 can also be fully processed by caspase-320 and depletion of caspase-3 from cell extracts blocked caspase-2 processing in vitro.31,32 This prompted us to test whether caspase-2 activation can occur in cells lacking Apaf-1 or caspase-9, that constitute one of the main caspase-3 activation mechanisms.
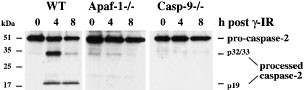
We isolated wt, Apaf-1−/− or caspase-9−/− thymocytes from lethally irradiated mice, reconstituted with fetal liver cells derived from wt, Apaf-1−/− or Caspase-9−/− E14 embryos. CD4+8+ thymocytes were purified by immunofluorescent staining and flow cytometric cell sorting, and exposed to 5 Gy γ-irradiation. After 0, 4 or 8 h in culture, cells were harvested, lysed and Western blotting performed with anti-caspase-2 antibodies to follow the appearance of the p32/33 and p19 cleavage products and the disappearance of the p51 pro-form, as a sign of caspase-2 processing. The caspase-2 cleavage products were apparent after γ-irradiation only in wt thymocytes (Figure 8) but not in those of Apaf-1−/− and caspase-9−/− origin (Figure 8). These results demonstrate that in primary cells the cleavage of caspase-2 occurs downstream of the Apaf-1/caspase-9 apoptosome complex and are in agreement with the finding that depletion of caspase-9 from cell extracts in vitro abrogates cytochrome _c_-inducible activation of caspase-2.31 Thus, Apaf-1 and caspase-9 are required for caspase-2 activation.
Figure 8
Caspase-2 processing is defective in thymocytes derived from Apaf-1 and caspase-9 deficient mice. Cell death was induced in WT, Apaf-1−/− or caspase-9−/− thymocytes by γ-irradiation (5 Gy) and cell lysates were prepared 0, 4 and 8 h after apoptosis induction. Caspase-2 processing was assessed by Western blotting using anti-caspase-2 mAb 11B4 and detected by ECL
Conclusions
Using monoclonal antibodies that recognize the p19 subunit of caspase-2 we have shown that caspase-2 is expressed in most tissues and cell types. Further, we demonstrate that caspase-2 is located in the nuclear and cytosolic compartments, in particular the Golgi apparatus. Although caspase-2 is activated in response to a broad range of apoptotic stimuli, it is not essential for apoptosis in many cell types, since thymocytes and DRG neuronal cells derived from caspase-2 mutant mice die normally. We also show, that caspase-2 processing during γ-irradiation induced cell death is dependent on the presence of Apaf-1 and caspase-9. This indicates that cleavage of caspase-2 occurs downstream of the Apaf-1/caspase-9 apoptosome and that caspase-2 processing may be dependent on other caspases, such as caspase-3. We have however seen that in MCF7 cells, that lack the caspase-3 gene,33 caspase-2 activation can occur in response to various death stimuli (data not shown). This suggests that caspase-3 mediated proteolysis may be only one of the ways of caspase-2 activation and in the absence of caspase-3, either an autocatalytic mechanism, or other caspases, such as caspase-7 can still mediate pro-caspase-2 processing. Collectively these observations indicate that in many circumstances caspase-2 may act as an effector caspase. It remains, however, possible that in certain other cell types, such as neuronal cells, caspase-2 can also function as an initiator caspase, but does so in an overlapping manner with another initiator caspase, such as caspase-9.34 To address the possible overlap between caspase-2 and caspase-9 function, it will therefore be interesting to generate mice lacking both of these enzymes.
Materials and Methods
Experimental animals
All experiments with animals were performed according to the guidelines laid down by the Royal Melbourne Hospital Research Foundation's Animal Ethics Committee. Wistar rats and C57BL/6 mice were obtained from The Walter and Eliza Hall Institute's breeding facility.
For the generation of caspase-2−/− mice the targeting construct (Figure 2) contained the gene for neomycin resistance under the control of the pgk promoter and a PGK-TK cassette for positive-negative selection. The neo cassette replaced a 1.65 kb fragment of the caspase-2 gene containing exon 6 (including the catalytic cysteine) and part of exon 7. The construct was linearised and electroporated into w9.5 embryonic stem cells that were selected using gancyclovir and G148 (neomycin). Antibiotic-resistant ES cell clones were isolated, expanded and screened by Southern blot hybridisation using a probe situated at the 3′ end of the deletion construct. ES cells targeted at the caspase-2 locus were confirmed to contain a single integration using a neomycin probe. These cells were used to derive chimaeric mice that were mated with C57BL/6 female mice and the heterozygous offsprings interbred to yield wild-type caspase-2+/+, heterozygous caspase-2+/−, and mutant caspase-2−/− mice. Mouse geneotypes were determined by Southern blot analysis of genomic DNA obtained from tail biopsies. Mice were maintained on a mixed 129/sv×C57BL/6 background. The generation of Apaf-1 mutant and caspase-9 mice has been described.35,36 Apaf-1+/− and caspase-9+/− animals were backcrossed for >8 generations with C57BL/6 mice prior to mating for generation of Apaf-1−/− and caspase-9−/− embryos.
Production and purification of the hexa-His-tagged Caspase-2 p19 polypeptides
A region of mouse caspase-2 encoding the p19 subunit (amino acids 167 to 333) was PCR amplified from pMSN2.411 and cloned into pQE30 vector (Qiagen) containing an amino terminal 6×His tag to generate pQEp19. Overnight E. coli M15(pREP4) cultures harbouring pQEp19 were diluted 50-fold in fresh medium, grown for 2 h at 37°C and induced with 1 mM IPTG for a further 4 h. Bacterial pellets were lysed in 8.0 M urea, 0.1 M NaH2PO4, 0.01 M Tris HCl, pH 8.0 and 6×His-tagged protein purified using a TALON column (Clontech). The recombinant protein was dialysed against PBS, precipitated by cold acetone and redissolved in PBS at 1 mg/mL.
Immunisation, hybridoma fusion and screening for antibodies to caspase-2
Wistar rats were initially immunised by subcutaneous (s.c.) injection with 100 μg purified recombinant 6×His tagged caspase-2 p19 subunit mixed with complete Freund's adjuvant (Difco). Two subsequent boosts of the immunogen, resuspended in incomplete Freund's adjuvant (Difco), were injected s.c. 3 and 6 weeks later. A final boost with p19 protein dissolved in PBS was given intravenously (i.v.) and intraperitoneally (i.p.) 4 weeks after this. Three days later, hybridomas were generated by fusing spleen cells from immunised rats with the SP2/0 myeloma cell line as previously described.22 Hybridomas producing monoclonal antibodies to caspase-2 were identified using a screening strategy that we have previously described.22 Briefly, 293T cells were transiently transfected with a C-terminally HA-tagged mutant of mouse caspase-2 in which the active site cysteine had been changed to alanine. Transfected cells were fixed in 1% paraformaldehyde, permeabilised with 0.3% saponin (Sigma) and stained with hybridoma supernatants. Bound antibodies were revealed with fluorescein-isothyocyanate (FITC)-conjugated goat anti-rat Ig antibodies (Southern Biotechnology) and analysed in a FACScan analyzer (Becton Dickinson). Hybridomas producing antibodies to caspase-2 were cloned twice and adapted for growth in medium containing low serum. For production of large amounts of antibodies, hybridomas were cultured for several weeks in the miniPERM classic 12.5 kDa production and nutrient module (Heraeus). Antibodies were purified on a protein-G Sepharose column (Pharmacia).
Cell lines, tissue culture, cell death assays and transfection with expression constructs
The cell lines used for analysis of caspase-2 expression are indicated in Table 1. Details of these cell lines are available from the authors. The cells were cultured in the high glucose version of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal calf serum (FCS), 50 μM 2-mercaptoethanol (2-ME), 13 μM folic acid and 100 μM L-asparagine or were grown in DME or RPMI medium with 10% FCS alone. Cultures of parental FDC-P1 and BAF-3 cells and their derivatives were supplemented with IL-3 (1000 U/mL). Granulocyte differentiation of 34.6 Myl cells was induced by the addition of 1.5% dimethyl sulfoxide.37 Liposome (Lipofectamine, Gibco BRL) mediated transfection of 293T cells was performed as previously described.38
Apaf-1−/− and caspase-9−/− thymocytes were isolated from C57BL/6-Ly5.1 mice, which had been lethally irradiated (2×5.5 Gy) and reconstituted with foetal liver cells from wild-type (wt), Apaf-1−/− or Caspase-9−/− E14 fetuses (all >8 generations C57BL/6-Ly5.2). These fetuses were generated by intercrosses of either Apaf-1+/−35 or Caspase-9+/− mice.36 Donor (Ly5.2+)-derived CD4+8+ thymocytes were purified by immunofluorescent staining with monoclonal antibodies to Ly5.2, CD4 and CD8, followed by cell sorting in a MoFlo (cytomation).39 These thymocytes were left untreated or were exposed to 5 Gy γ-irradiation. After 4 or 8 h in culture, cells were harvested, lysed and Western blotting performed with anti-caspase-2 antibodies.
In BAF-3 and FDC-P1 cells, apoptosis was induced by withdrawal of IL-3, their essential growth factor. Cell viability was determined by counting cells stained with the vital dye Trypan Blue.
Thymocytes from wild-type (wt) or caspase-2−/− mice were cultured in DMEM supplemented with 10% foetal calf serum (FCS), 50 μM 2-mercaptoethanol (2-ME), 13 μM folic acid and 100 μM L-asparagine at 106/ml. Cell death was induced by treatment with either 1–1000 ng/ml anti-Fas Ab (Jo2, Pharmingen), 1 μM dexamethasone (Sigma) or γ-irradiation (4–12 Gy). Cell viability was determined after 24 h by propidium iodide (PI) staining and analysis in a FACScan.
Dorsal root ganglion (DRG) neuronal cells from wt and caspase-2−/− mice were prepared as described40 from post-natal day 2 mice. Briefly, DRGs were dissected free of surrounding tissue and incubated in HEPES-buffered Eagles medium (HEM), 0.025% trypsin, 0.001% DNAse for 30 min. FCS was added (10%) and cells centrifuged at 300×g for 5 min, resuspended in HEM, 10% FCS, 0.01% DNAse and triturated through 18 to 24 gauge needles to obtain a single cell suspension. Cells were washed in HEM, 10% FCS, 0.01% DNAse and resupended in Monomed, 10% FCS. To assess DRG neuron survival, single cell suspensions were resupsended at a density of 104 cells/mL with or without added nerve growth factor (2.5S NGF, 10 ng/mL, Sigma). Cells were plated on fibronectin (Sigma) coated Terasaki plates at approximately 100 cells/well. At each time point, at least five wells were stained with MTT (0.5 mg/mL 3-(4,5-dimethythiazol-2,5-diphenyltetrazolium bromide, Sigma) for 1–2 h at 37°C and viable (blue stained cells) counted. This number was expressed as a percentage of the mean number of surviving cells at 0 h.
Western blotting
Primary cells, cell lines or transfected 293T cells were harvested, washed twice in cold PBS and lysed in lysis buffer (20 mM Tris/HCl, pH 8.0, 125 mM NaCl, 1 mM EGTA, 1% Triton X-100, 10% glycerol, 0.5 μg/mL Pefabloc, 1 μg/mL of each of leupeptin, aprotinin, soybean trypsin inhibitor and pepstatin, 5 mM NaF and 2 mM Na3VO4; all reagents from Sigma or Roche Diagnostics). For preparation of tissue lysates, organs were excised, washed in PBS, immediately frozen in isopentane on dry ice, and were later homogenised at 4°C in lysis buffer as described before.41
Proteins in cell lysates were size-fractionated by SDS–PAGE and electroblotted onto nitrocellulose membranes (Amersham LifeScience). Prior to immunoblotting, non-specific binding of antibodies to membranes was blocked by incubation overnight in 5% skimmed milk, 1% casein, 0.05% Tween-20. Membranes were probed with 10C6 or 11B4 anti-caspase-2 mAbs (1 μg/mL) followed by goat anti-rat IgG antibodies conjugated to HRP (Southern Biotechnology) and detection by enhanced chemiluminescence (ECL; Amersham Pharmacia). To control for the concentration and integrity of proteins in the tissue lysates, blots were probed with mouse anti-HSP70 mAb N6 (a gift from Dr R Anderson, Peter MacCallum Cancer Institute, Melbourne, Australia), followed by HRP-conjugated sheep anti-mouse Ig antibodies (Silenus) and detection by ECL.
Subcellular fractionation
Jurkat or HeLa cells were resuspended in hypotonic buffer (either 0.01 M NaCl, 1.5 mM MgCl2, 0.01 M Tris-HCl, pH 7.4 or 10 mM HEPES pH 7.4, 38 mM NaCl) and allowed to swell on ice for 10 min at 4°C. Cells were lysed using a Dounce homogeniser (12–36 strokes with a type ‘B’ pestle; Konte Glassware Corporation).42 After 3 min centrifugation at 1000×g at 4°C, followed by two washes in hypotonic buffer, the pelleted nuclei or heavy membranes (HM) were resuspended in the lysis buffer used for Western blotting (see above). The resulting supernatant was fractionated further by centrifugation at 130 000×g for 1 h (or 50 000 r.p.m. for 30 min in a TLA 100.3 Beckman benchtop rotor) to generate the soluble (S) and light membrane (LM) fractions. The LM pellet was resuspended in RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% deoxycholic acid, 50 mM Tris-HCl pH 8.0 plus protease inhibitors). Lysates from equivalent numbers of cells were analyzed by immunoblotting with the rat anti-caspase-2 mAbs 10C6 or 11B4, the mouse mAb anti-PARP (Calbiochem-Novabiochem Corp), rabbit anti-Golgi β-COP (Affinity Bioreagents Inc) or mouse anti-HSP-70 mAb. Bound antibodies were revealed with appropriate HRP-conjugated anti-mouse, anti-rat or anti-rabbit Ig antibodies and ECL detection.
Immunofluorescence staining and confocal microscopy
For immunofluorescence staining with anti-caspase-2 mAbs, HeLa cells were grown in chamber slides (Becton Dickinson). Cells were attached using Cell Tak (Becton Dickinson), fixed and permeabilized with PBS/4% paraformaldehyde, containing 0.18% Triton X-100 for 10 min at RT. The fixed cells were stained overnight at 4°C with the rat anti-caspase-2 10C6 or 11B4 mAbs in PBS containing 10% FCS, washed with PBS and then incubated with FITC-conjugated goat anti-rat Ig antibodies (Southern Biotechnology). The slides were then re-blocked with normal goat serum and stained rabbit anti-beta-Coatomer protein (β-COP) antibody (Affinty BioReagents, Inc) in PBS containing 10% FCS, followed by biotinylated goat anti-rabbit IgG antibodies (Vector) in PBS containing 10% FCS. Staining was detected by Texas Red conjugated strepavidin (Vector) containing DAPI (2 μg/mL, Molecular Probes). Slides were mounted in fluorescent mounting medium (Dako) containing 100 μg/mL DIABCO (Sigma). Controls included staining with an isotype matched rat IgG2a antibody (Pharmingen) or with the primary or secondary antibodies alone. Samples were analysed with a Leica confocal scanning microscope using SCANware software (Leica Lasertechnik).
Abbreviations
LM:
light membrane
HM:
heavy membrane
mAb:
monoclonal antibody
NGF:
Nerve Growth Factor
ECL:
enhanced chemical luminescence
zVAD-fmk:
benzyloxy-carbonyl-Val-Ala-Asp- fluoromethylketone
DEX:
dexamethasone
DRG:
dorsal root ganglion
References
- Nicholson DW . 1999 Caspase structure, proteolytic substrates, and function during apoptotic cell death Cell Death Differ. 6: 1028–1042
Article CAS Google Scholar - Kumar S, Colussi PA . 1999 Prodomains - adaptors - oligomerization: the pursuit of caspase activation in apoptosis Trends Biochem. Sci. 24: 1–4
Article CAS Google Scholar - Strasser A, O'Connor L, Dixit VM . 2000 Apoptosis signaling Annu. Rev. Biochem. 69: 217–245
Article CAS Google Scholar - Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D . 1995 A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain J. Biol. Chem. 270: 7795–7798
Article CAS Google Scholar - Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM . 1995 FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis Cell 81: 505–512
Article CAS Google Scholar - Boldin MP, Goncharov TM, Goltsev YV, Wallach D . 1996 Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death Cell 85: 803–815
Article CAS Google Scholar - Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM . 1996 FLICE, a novel FADD homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex Cell 85: 817–827
Article CAS Google Scholar - Salvesen GS, Dixit VM . 1999 Caspase activation: the induced-proximity model Proc. Natl. Acad. Sci. USA 96: 10964–10967
Article CAS Google Scholar - Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X . 1997 Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade Cell 91: 479–489
Article CAS Google Scholar - Zou H, Henzel WJ, Liu X, Lutschg A, Wang X . 1997 Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of Caspase-3 Cell 90: 405–413
Article CAS Google Scholar - Kumar S, Kinoshita M, Noda M, Copeland NG, Jenkins NA . 1994 Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1β-converting enzyme Genes Dev. 8: 1613–1626
Article CAS Google Scholar - Kumar S, Kinoshita M, Dorstyn L, Noda M . 1997 Origin, expression and possible functions of the two alternatively spliced forms of the mouse Nedd2 mRNA Cell Death Differ. 4: 378–387
Article CAS Google Scholar - Troy CM, Stefanis L, Greene LA, Shelanski ML . 1997 Nedd2 is required for apoptosis after trophic factor withdrawal, but not superoxide dismutase (SOD1) downregulation, in sympathetic neurons and PC12 cells J. Neurosci. 17: 1911–1918
Article CAS Google Scholar - Nicholson DW, Thornberry NA . 1997 Caspases: killer proteases Trends Biochem. Sci. 22: 299–306
Article CAS Google Scholar - Duan H, Dixit VM . 1997 RAIDD is a new ‘death’ adaptor molecule Nature 385: 86–89
Article CAS Google Scholar - Shearwin-Whyatt LM, Harvey NL, Kumar S . 2000 Subcellular localization and CARD-dependent oligomerization of the death adaptor RAIDD Cell Death Differ. 7: 155–165
Article CAS Google Scholar - Harvey NL, Butt AJ, Kumar S . 1997 Functional activation of Nedd2/ICH-1 (caspase-2) is an early process in apoptosis J. Biol. Chem. 272: 13134–13139
Article CAS Google Scholar - Butt AJ, Harvey NL, Parasivam G, Kumar S . 1998 Dimerization and autoprocessing of the Nedd2 (caspase-2) precursor requires both the prodomain and the carboxyl-terminal regions J. Biol. Chem. 273: 6763–6768
Article CAS Google Scholar - Colussi PA, Harvey NL, Kumar S . 1998 Prodomain-dependent nuclear localization of the caspase-2 (Nedd2) precursor. A novel function for a caspase prodomain J. Biol. Chem. 273: 24535–24542
Article CAS Google Scholar - Harvey NL, Trapani JA, Fernandes-Alnemri T, Litwack G, Alnemri ES, Kumar S . 1996 Processing of the Nedd2 precursor by ICE-like proteases and granzyme B. Genes Cells 1: 673–685
Article CAS Google Scholar - Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC, Hara H, Moskowitz MA, Li E, Greenberg A, Tilly JL, Yuan J . 1998 Defects in regulation of apoptosis in caspase-2-deficient mice Genes Dev. 12: 1304–1314
Article CAS Google Scholar - O'Reilly LA, Cullen L, Moriishi K, O'Connor L, Huang DCS, Strasser A . 1998 Rapid hybridoma screening method for the identification of monoclonal antibodies to low abundance cytoplasmic proteins BioTechniques 25: 824–830
Article CAS Google Scholar - Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA . 1998 Inhibition of human caspases by peptide-based and macromolecular inhibitors J. Biol. Chem. 273: 32608–32613
Article CAS Google Scholar - Kumar S, Kinoshita M, Noda M . 1997 Characterization of a mammalian cell death gene Nedd2 Leukemia 11 (Suppl 3): 385–386
PubMed Google Scholar - Droin N, Rébé C, Bichat F, Hammann A, Bertrand R, Solary E . 2001 Modulation of apoptosis by procaspase-2 short isoform: selective inhibition of chromatin condensation, apoptotic body formation and phosphatidylserine externalization Oncogene 20: 260–269
Article CAS Google Scholar - Zhivotovsky B, Samali A, Gahm A, Orrenius S . 1999 Caspases: their intracellular localization and translocation during apoptosis Cell Death Differ. 6: 644–651
Article CAS Google Scholar - Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prevost M-C, Alzari PM, Kroemer G . 1999 Mitochondrial release of caspase-2 and -9 during the apoptotic process J. Exp. Med. 189: 381–394
Article CAS Google Scholar - Mancini M, Machamer CE, Roy S, Nicholson DW, Thornberry NA, Casciola-Rosen LA, Rosen A . 2000 Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis J. Cell Biol. 149: 603–612
Article CAS Google Scholar - Hsu YT, Youle RJ . 1997 Nonionic detergents induce dimerization among members of the Bcl-2 family J. Biol. Chem. 272: 13829–13834
Article CAS Google Scholar - Kumar S . 1995 Inhibition of apoptosis by the expression of antisense Nedd2 FEBS Lett. 368: 69–72
Article CAS Google Scholar - Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ . 1999 Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9- dependent manner J. Cell Biol. 144: 281–292
Article CAS Google Scholar - Paroni G, Henderson C, Schneider C, Brancolini C . 2001 Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3 J. Biol. Chem. 276: 21907–21915
Article CAS Google Scholar - Janicke RU, Sprengart ML, Wati MR, Porter AG . 1998 Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis J. Biol. Chem. 273: 9357–9360
Article CAS Google Scholar - Troy CM, Rabacchi SA, Hohl JB, Angelastro JM, Greene LA, Shelanski ML . 2001 Death in the balance: alternative participation of the caspase-2 and -9 pathways in neuronal death induced by Nerve Growth Factor deprivation J. Neurosci. 21: 5007–5016
Article CAS Google Scholar - Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P . 1998 Apaf-1 (CED-4 homologue) regulates programmed cell death in mammalian development Cell 94: 727–737
Article CAS Google Scholar - Kuida K, Haydar TF, Kuan C-Y, Gu Y, Taya C, Karasuyama H, Su MS-S, Rakic P, Flavell RA . 1998 Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9 Cell 94: 325–337
Article CAS Google Scholar - Elefanty AG, Cory S . 1992 _bcr-abl_-Induced cell lines can switch from mast cell to erythroid or myeloid differentiation in vitro Blood 79: 1271–1281
CAS PubMed Google Scholar - Huang DCS, O'Reilly LA, Strasser A, Cory S . 1997 The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry EMBO J. 16: 4628–4638
Article CAS Google Scholar - O'Reilly LA, Huang DCS, Strasser A . 1996 The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry EMBO J. 15: 6979–6990
Article CAS Google Scholar - Murphy M, Reid K, Hilton DJ, Bartlett PF . 1991 Generation of sensory neurons is stimulated by leukemia inhibitory factor Proc. Natl. Acad. Sci. USA 88: 3498–3501
Article CAS Google Scholar - Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Köntgen F, Adams JM, Cory S . 1998 Apoptosis regulator Bcl-w is essential for spermatogenesis but appears otherwise redundant Proc. Natl. Acad. Sci. USA 95: 12424–12431
Article CAS Google Scholar - Hausmann G, O'Reilly LA, van Driel R, Beaumont JG, Strasser A, Adams JM, Huang DCS . 2000 Pro-apoptotic apoptosis protease-activating Factor 1 (Apaf-1) has a cytoplasmic localization distinct from Bcl-2 or Bcl-xL J. Cell Biol. 149: 623–634
Article CAS Google Scholar
Acknowledgements
We are grateful to Prof. Peter Gruss (Max Plank Institute, Goettingen, Germany) for supplying the Apaf-1+/− mice. We thank S Novakovic, G Hausmann, F Battye, D Kaminaris, J Parker, V Lapatis, C Tarlinton, A Milligan and J Merryfull for help with various aspects of this study. This work was supported by grants and fellowships from, the Leukemia and Lymphoma Society of America (New York), the Anti-Cancer Foundation of South Australia, the Anti-Cancer Council of Victoria, the National Health and Medical Research Council, the Sylvia and Charles Viertel Charitable Foundation, the Dr. Josef Steiner Cancer Research Foundation (Bern, Switzerland) and The Murdoch Children's Research Institute.
Author information
Author notes
- G Hacker
Present address: Technical University of Munich, Munich, Germany - C Magnusson
Present address: Pharmacia, Uppsala, Sweden
Authors and Affiliations
- The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia
L A O'Reilly, P Ekert, V Marsden, L Cullen, D L Vaux, G Hacker, C Magnusson, M Pakusch, A Strasser & D C S Huang - The Hanson Centre for Cancer Research, Institute of Medical and Veterinary Science, Adelaide, Australia
N Harvey & S Kumar - Murdoch Children's Research Institute, Melbourne, Australia
P Ekert - University of Rome ‘Tor vergata’, Rome, Italy
F Cecconi - Vertex Pharmaceuticals, Cambridge, MA, USA
K Kuida
Authors
- L A O'Reilly
You can also search for this author inPubMed Google Scholar - P Ekert
You can also search for this author inPubMed Google Scholar - N Harvey
You can also search for this author inPubMed Google Scholar - V Marsden
You can also search for this author inPubMed Google Scholar - L Cullen
You can also search for this author inPubMed Google Scholar - D L Vaux
You can also search for this author inPubMed Google Scholar - G Hacker
You can also search for this author inPubMed Google Scholar - C Magnusson
You can also search for this author inPubMed Google Scholar - M Pakusch
You can also search for this author inPubMed Google Scholar - F Cecconi
You can also search for this author inPubMed Google Scholar - K Kuida
You can also search for this author inPubMed Google Scholar - A Strasser
You can also search for this author inPubMed Google Scholar - D C S Huang
You can also search for this author inPubMed Google Scholar - S Kumar
You can also search for this author inPubMed Google Scholar
Corresponding authors
Correspondence toL A O'Reilly or S Kumar.
Additional information
Edited by S J Martin
Rights and permissions
About this article
Cite this article
O'Reilly, L., Ekert, P., Harvey, N. et al. Caspase-2 is not required for thymocyte or neuronal apoptosis even though cleavage of caspase-2 is dependent on both Apaf-1 and caspase-9.Cell Death Differ 9, 832–841 (2002). https://doi.org/10.1038/sj.cdd.4401033
- Received: 29 November 2001
- Revised: 28 January 2002
- Accepted: 08 February 2002
- Published: 16 July 2002
- Issue Date: 01 August 2002
- DOI: https://doi.org/10.1038/sj.cdd.4401033