Functional characterization of the Bcl-2 gene family in the zebrafish (original) (raw)
Introduction
Members of the Bcl-2 gene family are critical mediators of the delicate balance between survival and apoptosis in eucaryotic cells.1 Dysregulation of this signaling pathway can result in a myriad of diseases, including cancer, autoimmune diseases and degenerative disorders.2, 3 The Bcl-2 family consists of three classes of proteins: prosurvival (including Bcl-2, Bcl-xL, Mcl-1, A1, Boo/DIVA, Bcl-B, and Bcl-w), multidomain proapoptotic (including Bax, Bak, and Bok), and proapoptotic BH3 only (Bid, Bad, Bmf, Bik, Puma, Noxa, Hrk, and BNip3).4 In unstimulated cells, interactions between prosurvival and proapoptotic multidomain family members prevent the latter from oligomerizing and initiating the apoptotic program.5 Upon apoptotic stimulation, BH3-only proteins relieve the inhibition of multidomain proapoptotic proteins, freeing them to initiate apoptosis.6, 7 The subsequent release of proapoptotic factors from the mitochondria results in the stimulation of caspase-9, and cleavage and activation of effector caspases such as caspase-3, -6, and -7.
Previous experiments demonstrate that several members of the Bcl-2 family are required for normal development; however, the function of this protein family during development is largely unknown. Simultaneous knockout of Bax and Bak results in drastically decreased perinatal survival, with fewer than 10% of the double-knockout mice surviving to adulthood. These mice are born in normal Mendelian ratios, but are neglected by the mother and likely die from failure to nurse,8 perhaps as a result of massive inappropriate neuronal accumulation. Bcl-x knockouts reveal a critical role for Bcl-x L in the survival of developing neuronal and hematopoietic cell types,9 whereas the Bcl-2 knockout has only moderate effects in adult mice.10, 11, 12 Knockout of Mcl-1 results in preimplantation lethality.13 While these experiments clearly indicate important roles for Bcl-2 family members in early development, in each of these knockouts neither the initiating apoptotic signal nor the activated BH3-only proteins are known.
We have therefore characterized the complement of Bcl-2 family proteins in zebrafish. This genetically tractable model organism offers the unique opportunity to elucidate the role of the Bcl-2 genes during embryogenesis ex utero. While the existence of several Bcl-2 family members in zebrafish has been reported,14, 15 little is known about the functional homology between zebrafish and mammalian Bcl-2 family members. Here, we report that the zebrafish genome contains full-length, functional homologs of most Bcl-2 family members. Overexpression of homologs of most mammalian multidomain or BH3-only proapoptotic genes results in apoptosis. Coexpression of homologs of mammalian prosurvival Bcl-2 family members can rescue this death. Furthermore, we show that DNA damage induced by _γ_-irradiation leads to a massive, _p53_-dependent upregulation of zPuma. Induction of the apoptotic response to _γ_-irradiation is dependent on zebrafish homologs of mammalian Bax, and ectopic expression of any zebrafish homolog of mammalian prosurvival Bcl-2 family member is sufficient to prevent irradiation-induced apoptosis. In addition, we show that endogenous zMcl-1a and zMcl-1b, but not the Bcl-2 homolog zBlp2, play an important role in maintaining the health of early zebrafish embryos. Importantly, we also demonstrate that zMcl-1a and zMcl-1b are specifically required to protect embryos from death ligand (DL)-induced apoptosis early in development, indicating that the intrinsic pathway confers sensitivity to death receptor-mediated apoptosis. Thus, the zebrafish provides a promising model to investigate the function of Bcl-2 family members in nontransformed vertebrate tissues and during normal embryogenesis.
Results
Zebrafish genome contains homologs of most mammalian Bcl-2 family members
We have identified and functionally characterized the complement of full-length zebrafish Bcl-2 family members, including homologs of mammalian prosurvival Bcl-x L, Bcl-2, Mcl-1, and Boo/DIVA; proapoptotic Bax and Bok; and BH3-only genes Bid, Bad, Bmf, Noxa, Puma, and Bik. Previous reports have described zebrafish homologs to a subset of mammalian Bcl-2 family genes, including Bcl-2, Bcl-x L, Mcl-1, NR13, and Bad.16, 17, 18, 19 Furthermore, ESTs bearing homology to proapoptotic Bcl-2 family members Bax, BNip3, Bmf, Noxa, and Bid have been identified.14, 15 Here, we present the first comprehensive evaluation of this gene family in zebrafish.
Because of genomic duplication in the teleost lineage,20 several mammalian Bcl-2 family genes have two copies in zebrafish, including Mcl-1, Bmf, Bok, and Bax (Table 1). Regions sharing conserved synteny with human Mcl-1 chromosome locus 1q21.2 are present in two zebrafish chromosomes; thus, despite being in separate chromosomal locations, both zebrafish copies of Mcl-1 have a conserved syntenic relationship with the human counterpart. Similarly, both zBok1 and zBok2 share conserved synteny with human Bok despite the zebrafish genes mapping to different chromosomes. In contrast, zBmf2, but not zBmf1, has conserved synteny with its mammalian homolog; zBax2, but not zBax1, shares conserved synteny with human Bax. zBlp1, zNR13, zBad, zBik, and zPuma all share a conserved syntenic relationship with their human counterparts (Table 1).
Table 1 Bcl-2 family is strongly conserved between zebrafish and human species
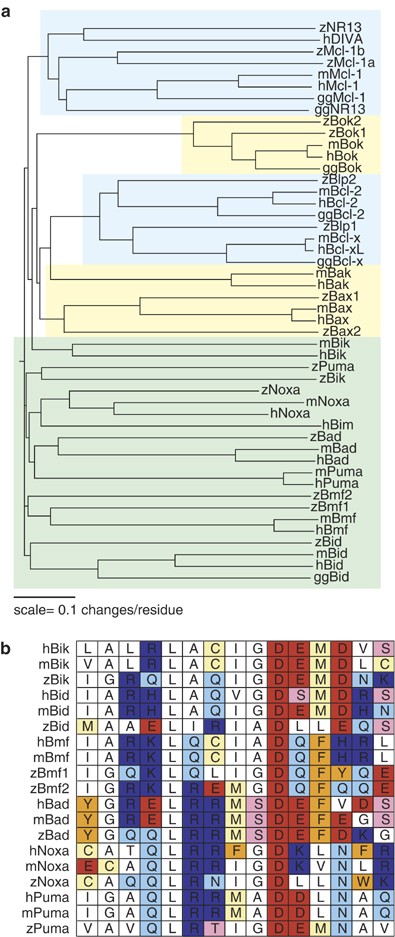
Overall, the multidomain Bcl-2 family members bear the highest degree of homology to their mammalian counterparts (Figure 1a). Alignment of the amino-acid sequence reveals that each of the zebrafish homologs of mammalian prosurvival Bcl-2 genes cluster most closely with their mammalian homologs, as do zBok1, zBok2, zBax1, and zBax2.
Figure 1
Homologs of the majority of mammalian Bcl-2 family members are present in zebrafish. (a) Zebrafish Bcl-2 family members are aligned with their human (h), mouse (m), and (where available) chicken (gg) homologs. Prosurvival genes are highlighted in blue, multidomain proapoptotic genes are highlighted in yellow, and BH3-only genes are highlighted in green. (b) The BH3 domains of human, mouse, and zebrafish BH3-only proteins are grouped according to gene
Zebrafish homologs of Noxa, Bad, Bmf, and Bid all cluster with their mammalian counterparts, yet they share only distant identity (Figure 1a). While the overall amino-acid sequence of zebrafish BH3-only genes has diverged significantly from mammalian counterparts, the critical BH3 domain retains a higher degree of homology (Figure 1b), highlighting the functional importance of this domain.
Zebrafish Bik and Puma are the most divergent of the zebrafish BH3-only genes. However, both share conserved synteny with the human sequence (Table 1), and in the case of zPuma, the critical BH3 domain aligns most closely with the mammalian homolog (Figure 1b). In contrast, the BH3 domain of zBik bears a striking resemblance to the corresponding domain of human and mouse Bid, rather than mammalian Bik (Figure 1b). However, as zBik lacks a predicted caspase cleavage site and is syntenic with human Bik, it is clearly the zebrafish ortholog of human Bik.
While we initially identified zebrafish Bcl-2 family genes on the basis of Bcl-2-homology (BH) domain structure, percent homology, and synteny with human homologs, we examined additional sequence features to confirm homolog identity when possible. Alignments between zBad and its human ortholog revealed that residues Ser112 and Ser136 are highly conserved: phosphorylation of these serines in mammalian Bad facilitates binding to 14-3-3, regulating its proapoptotic activity.21 Similarly to human Bid,22 zBid has a putative caspase-8 cleavage site, ostensibly allowing generation of a tBid-like fragment. In addition, zBmf1 and zBmf2 contain putative dynein light-chain-binding domains: mammalian Bmf is regulated via dynein light-chain-mediated sequestration to the actin cytoskeleton until activation.23 These findings suggest that several Bcl-2 family members may be regulated by similar mechanisms in zebrafish and mammals. Zebrafish homologs of Bcl-B, Bcl-w, A1, and Hrk have yet to be identified.
Zebrafish Bcl-2 family members are expressed throughout early embryonic development and in adult tissues
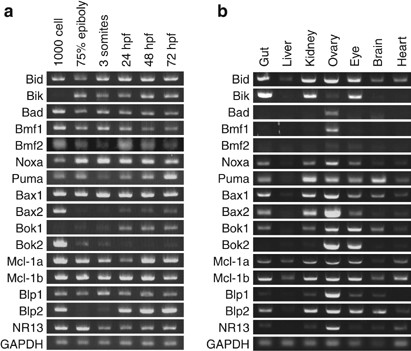
To assess whether any zebrafish Bcl-2 family members are present during embryonic development, we analyzed their expression by stage-specific RT-PCR (Figure 2a). We detected maternal expression of all of the Bcl-2 family members, except zBik and zBok1 (1000-cell stage). After the initiation of zygotic transcription, the levels of zBid, zBik, zBad, zBmf1, zNoxa, zBax1, zMcl-1a, zMcl-1b, zBlp1, and zNR13 were fairly constant. Conversely, the transcription of zBax2, zBmf2, and zBlp2 decreased dramatically after the 1000-cell stage before increasing again later in development.
Figure 2
Homologs of mammalian Bcl-2 family members are expressed in zebrafish. (a) Stage-specific RT-PCR reveals that most zebrafish Bcl-2 family members are expressed at readily detectable levels from 1000-cell stage up until 72 h.p.f. Exceptions are zBik and zBok1, which are absent maternally and are turned on later in development; zBax2 and zBlp2, which are present maternally and later in development; and zBok2, which is strongly expressed maternally but is largely absent later in embryonic development. GAPDH is included as a control for the amount of input RNA. (b) Zebrafish Bcl-2 family members are expressed specifically in adult tissues. The ovary is especially rich in Bcl-2 family members, whereas brain and liver express selected Bcl-2 family genes at much lower levels. GAPDH is included as a control for the amount of input RNA
Next, we asked whether the expression of Bcl-2 family members is tissue specific in adult zebrafish (Figure 2b). The zebrafish ovary was especially rich in Bcl-2 family members, expressing all genes. In contrast, the liver only weakly expressed zBid, zBax1, zMcl-1a, and zMcl-1b. Several of the zebrafish BH3-only genes, including zBik, zBad, zPuma, and zBmf1, showed a remarkably specific expression pattern at the RNA level: for example, zBik was highly expressed in the gut, kidney, and eye, but was absent in the liver, brain, and heart, whereas zPuma was enriched in brain tissue.
Ectopic expression of zebrafish homologs of mammalian proapoptotic Bcl-2 family members induces apoptosis
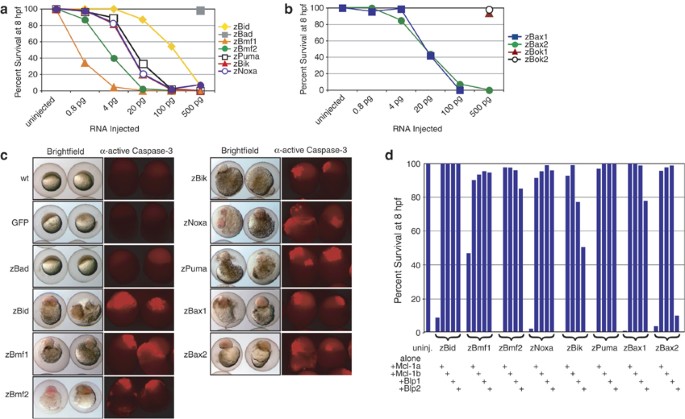
We next sought to determine whether the structural conservation between mammalian proapoptotic Bcl-2 family members and corresponding zebrafish homologs translated into conservation of in vivo function. Injection of mRNA encoding zebrafish homologs of mammalian proapoptotic BH3-only or multi-BH domain Bcl-2 family members triggered dose-dependent cell death, characterized by disintegration of the blastomeres and yolk cell (Figure 3a and b). Exceptions were zBad, zBok1, and zBok2, which did not induce significant apoptosis in early embryos even at high mRNA doses.
Figure 3
zBcl-2 family members are functional in embryonic zebrafish. Ectopic expression of BH3-only (a) and multi-BH domain (b) proapoptotic zBcl-2 family members induces death in a dose-dependent manner. Data are compiled from a minimum of two independent experiments per data point. (c) This death is defined by embryonic morphology at 8 h.p.f. (left panels), and by caspase-3 activation, which was detected by whole-mount immunohistochemistry at 3–5 h.p.f. (right panels). Embryos were injected with 500 pg GFP or zBad mRNA, or a minimally lethal dose of each proapoptotic mRNA. Each panel is representative of a minimum of two independent experiments. (d) Coexpression of prosurvival zBcl-2 family members inhibits death induced by overexpression of proapoptotic zBcl-2 proteins. Coexpression of zBlp2 cannot inhibit death induced by zBax2 overexpression, and can only weakly inhibit apoptosis induced by enforced expression of zBik. Five hundred picograms of mRNA of each prosurvival Bcl-2 family member and a minimally lethal dose of mRNA of each proapoptotic Bcl-2 family member were injected. Data are compiled from a minimum of two independent experiments per data point
To confirm that these genes induced cell death by engaging the apoptosis pathway, we injected embryos with a minimum lethal dose of each mRNA and analyzed proteolytic activation of effector caspase-3 by immunohistochemistry (Figure 3c). Compared to untreated and mock-injected embryos, overexpression of the death-inducing Bcl-2 family proteins resulted in a dramatic increase in activated caspase-3. Again, the exceptions were zBad, zBok1, and zBok2, which did not trigger readily detectable caspase-3 activation upon ectopic expression (Figure 3c, and data not shown). It is unclear why these homologs of mammalian proapoptotic genes did not induce death when overexpressed in zebrafish embryos; perhaps the encoded proteins require a specific cellular context (e.g. specific post-translational modification, such as dephosphorylation in the case of Bad) to engage the apoptotic program.
Ectopic expression of zebrafish homologs of mammalian prosurvival Bcl-2 family members inhibits intrinsic pathway-mediated apoptosis
Apoptosis induced by overexpression of a minimum lethal dose of zBid, zBmf1, zBmf2, zPuma, zNoxa, or zBax1 could be rescued by coexpression of zMcl-1a, zMcl-1b, zBlp1, or zBlp2 (Figure 3d). These data suggest that upon overexpression in zebrafish embryos, the proapoptotic genes induced cell death via a mechanism that resembles the mammalian apoptotic pathway. In contrast to zBlp1, zMcl-1a, or zMcl-1b, overexpressed zBlp2 failed to inhibit apoptosis triggered by ectopic expression of zBax2 (Figure 3d). These data suggest that zBax2 does not interact with the zebrafish homolog of mammalian Bcl-2. Similarly, human Bak interacts preferentially with Bcl-xL and Mcl-1, but not with Bcl-2.24 These results imply that zBax2, although orthologous to human Bax, has evolved similar selectivity to human Bak. Furthermore, zBlp2 could inhibit apoptosis triggered by overexpression of zBik only poorly, indicating that zBik interacts only weakly with zBlp2. Together, these findings suggest functional as well as structural conservation between mammalian and zebrafish Bcl-2 family genes, and support the existence of selective interactions between certain prosurvival and proapoptotic family members.
_γ_-Irradiation engages the intrinsic apoptosis pathway in zebrafish
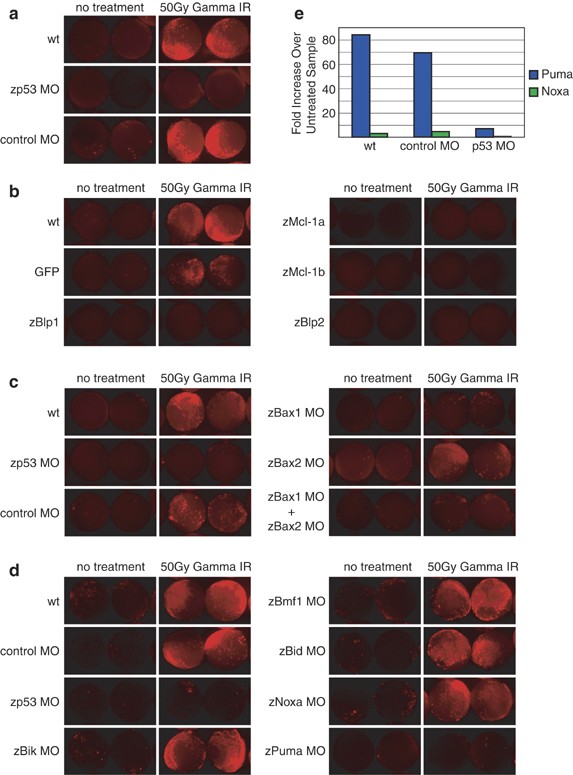
To investigate the role of Bcl-2 family members in the response to DNA damage, a well-established activator of the intrinsic apoptotic pathway, we subjected embryos to _γ_-irradiation. Exposure to _γ_-irradiation induced a substantial increase in activated caspase-3, which was dependent on p53 (Figure 4a).25 Ectopic expression of prosurvival Bcl-2 family members, such as zMcl-1a, zMcl-1b, zBlp1, or zBlp2, protected embryos from _γ_-irradiation-induced apoptosis (Figure 4b). Thus, Bcl-2 family genes control activation of the intrinsic pathway in response to _γ_-irradiation in zebrafish as well as in mammals.26
Figure 4
_γ_-Irradiation induces caspase-3 activation via the intrinsic apoptotic pathway in a _p53_-dependent manner. (a) Uninjected embryos, embryos injected with a p53 MO, and embryos injected with a control MO were subjected to 50 Gy of _γ_-irradiation at 7 h.p.f. and stained for caspase-3 activity at 10 h.p.f. _γ_-Irradiation induction of caspase-3 activity is _p53_-dependent. Each panel is representative of at least two independent experiments. (b) Injection of 500 pg zMcl-1a, zMcl-1b, zBlp1, or zBlp2 could efficiently inhibit caspase-3 activation in _γ_-irradiated embryos. Each panel is representative of at least two independent experiments. (c) Knockdown of zBax1, but not zBax2, could inhibit _γ_-irradiation-induced caspase-3 activation. Each panel is representative of four independent experiments. (d) Knockdown of zPuma, but not knockdown of any other BH3-only protein, inhibited caspase-3 activation in _γ_-irradiated zebrafish embryos. Each panel is representative of at least two independent experiments. (e) Taqman analysis reveals that zPuma is massively upregulated in _γ_-irradiated embryos in comparison to untreated (control) embryos. Expression of zNoxa was upregulated to a much lesser extent. _γ_-Irradiation-induced upregulation of both zNoxa and zPuma is dependent on p53
In most mammalian cell types, Bax and/or Bak are necessary and sufficient to trigger apoptosis activation through the intrinsic pathway.27 To determine whether zBax1 and zBax2 are functionally redundant in zebrafish, we injected morpholino oligonucleotides (MOs) directed against zBax1 and zBax2 individually or together and then subjected embryos to _γ_-irradiation (Figure 4c). Knockdown of zBax1 was sufficient to ameliorate the effects of _γ_-irradiation in four out of five experiments (_n_=∼30 embryos per condition in each experiment, Supplementary Figure 1). In the fifth experiment, knockdown of both zBax1 and zBax2 was required to abrogate _γ_-irradiation-induced caspase-3 activation (data not shown). Some caspase-3 activity remained detectable in the irradiated embryos injected with zBax1 and zBax2 MOs: this is probably a consequence of either incomplete knockdown or the function of residual maternal proteins present in the embryo (see Figure 2a). These data suggest that zBax1 plays a more important role than zBax2 in mediating activation of the apoptotic program in response to _γ_-irradiation, although both proteins support this function.
Next, we examined which BH3-only protein(s) is (are) critical for _γ_-irradiation-induced apoptosis in zebrafish embryos. MO knockdown of zBik, zBmf1, zBid, or zNoxa did not diminish caspase-3 activation in response to _γ_-irradiation. In contrast, knockdown of zPuma attenuated caspase-3 activation as effectively as did knockdown of p53 (Figure 4d), suggesting that this BH3-only protein is the primary initiator of this apoptotic response. Furthermore, quantitative RT-PCR analysis revealed that while _γ_-irradiation induced a three- to four-fold upregulation in zNoxa, it augmented zPuma expression almost 100-fold (Figure 4e). No other BH3-only gene showed increased transcription in response to _γ_-irradiation (data not shown). These data support previous findings in mammalian cells, indicating that Puma is the principal initiator of _γ_-irradiation-induced apoptosis.26, 28 Upregulation of zPuma was _p53_-dependent, reinforcing the importance of p53 and its transcriptional link to zPuma for apoptosis induction in response to DNA damage. Activation of zPuma may in turn lead to the activation of zBax1 and zBax2, an effect that we were able to antagonize by overexpression of zBlp1, zBlp2, and zMcl-1a and zMcl-1b.
Knockdown of zMcl-1a and zMcl-1b, but not zBlp2, decreases viability of zebrafish embryos
Having established that the Bcl-2 protein family is present and functional in zebrafish, we examined whether prosurvival family members play similar roles in zebrafish embryogenesis as they do in mice. To this end, we used MOs against zMcl-1a, zMcl-1b, and zBlp2. Knockdown of zBlp2 had no obvious effect on early zebrafish development. The gross morphology of embryos injected with zBlp2 MO was normal, and caspase-3 activity showed no detectable increase (data not shown). These data are reminiscent of the _Bcl_-2-knockout mice, which lack an embryonic phenotype,10 and suggest that the Bcl-2 homolog is dispensable for normal early embryonic development in vertebrates.
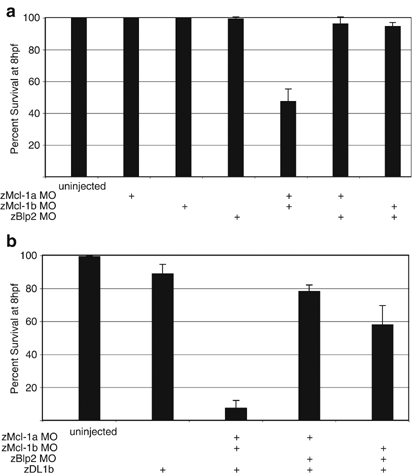
Knockdown of either zMcl-1a or zMcl-1b alone did not increase embryonic mortality (Figure 5a). In contrast, knockdown of both zMcl-1a and zMcl-1b resulted in a variable but significant decrease in viability by 8 hpf, suggesting that together the two zMcl-1 proteins are critical for normal embryonic development. These results agree with the knockout of mouse Mcl-1, which causes preimplantation lethality.13
Figure 5
Simultaneous knockdown of zMcl-1a and zMcl-1b increases embryonic mortality, and sensitizes embryos to DL signaling. (a) Knockdown of zMcl-1a, zMcl-1b, or zBlp2 alone has no effect on embryonic viability. Combined knockdown of zMcl-1a and zMcl-1b decreases embryonic viability by ∼40%. Knockdown of zBlp2 in combination with either zMcl-1a or zMcl-1b has no effect on embryonic viability. Error bars represent S.E.M. Data are compiled from a minimum of three independent experiments. (b) Knockdown of zMcl-1a and zMcl-1b sensitizes embryos to apoptosis induced by DL1b signaling. In contrast, knockdown of zBlp2 in combination with zMcl-1a or zMcl-1b sensitizes embryos to DL1b-induced apoptosis only to a minor extent. Error bars represent S.E.M. Data are compiled from a minimum of three independent experiments
It is formally possible that the lethality caused by combined knockdown of zMcl-1a and zMcl-1b is not specific to these genes; rather, knockdown of any two pro-survival genes could affect early zebrafish viability. To examine this possibility, we assessed the effect of pair-wise knockdown of different prosurvival Bcl-2 family members on embryonic viability (Figure 5a). Combined knockdown of zMcl-1a and zMcl-1b significantly affected the survival of injected embryos, while knockdown of zMcl-1a plus zBlp2 or zMcl-1b plus zBlp2 had little or no effect. Thus, zMcl-1a and zMcl-1b specifically are critical to normal early zebrafish development. Together, these results suggest that prosurvival Bcl-2 family members and particularly Mcl-1 have conserved functions during early development in vertebrates.
Knockdown of zMcl-1a and zMcl-1b, but not zBlp2, sensitizes zebrafish embryos to apoptosis induction through the extrinsic pathway
In certain mammalian cell types, apoptosis induction through the extrinsic pathway requires augmentation of the cell death signal through the intrinsic apoptotic pathway.29 Apo2L/TRAIL is an evolutionarily conserved proapoptotic DL of the extrinsic pathway that has several homologs in zebrafish, including zDL1b (Eimon et al., accompanying manuscript). Apoptosis signaling by zDL1b can be blocked by overexpression of prosurvival zBcl-2 family members (Eimon et al., accompanying manuscript). However, it is unknown which endogenous prosurvival Bcl-2 family members in zebrafish may function as inhibitors of extrinsic pathway augmentation. Therefore, we sought to determine whether knockdown of specific prosurvival Bcl-2 genes could sensitize embryos to apoptosis induction by zDL1b. In wild-type embryos, ectopic expression of zDL1b had minimal effect on early embryonic viability. However, upon combined knockdown of zMcl-1a and zMcl-1b, overexpression of zDL1b caused rapid and massive apoptotic death. This effect was specific to zMcl-1a and zMcl-1b: combining either zMcl-1a or zMcl-1b MO with zBlp2 MO showed only a minimal increase in sensitivity to _zDL1b_-induced apoptosis (Figure 5b). These data agree with the observation that in several mammalian cell lines, Mcl-1 specifically modulates sensitivity to _Apo2L/TRAIL_-induced apoptosis.30, 31, 32 Thus, Mcl-1 may be a key modulator of DL-induced apoptosis in cells that require engagement of both the extrinsic and intrinsic pathways for commitment to apoptosis. Furthermore, these results indicate that endogenous zMcl-1a and zMcl-1b play an important role in curtailing apoptosis induction through the cell-intrinsic pathway in early zebrafish embryos.
Discussion
Our results show that the zebrafish genome contains full-length, functional homologs of virtually all the mammalian Bcl-2 family members. Zebrafish offers a unique model to elucidate in greater detail the role of the various Bcl-2 family members during embryogenesis ex utero in a genetically tractable vertebrate. Although the existence of some zebrafish Bcl-2 family members has been reported previously,14, 15 several others were not known, and the functional relationship between zebrafish Bcl-2 family members has not been systematically elucidated.
By amino-acid sequence comparison, we identified homologs of most mammalian Bcl-2 family members, including BH3-only and multi BH domain proteins. The BH3-only family members are more divergent between zebrafish and mammals, but they share a high degree of homology within the critical BH3 domain. In both ectopic expression and MO knockdown experiments, the function of the majority of zebrafish Bcl-2 family members as proapoptotic or prosurvival regulators was fully consistent with the established function of their corresponding mammalian homologs. These findings demonstrate a remarkable conservation of the structure–function relationship of each Bcl-2 family member in vertebrate evolution.
zBax2 presented an intriguing exception to this stringent structure–function conservation. Even though it displays significantly greater sequence homology to mammalian Bax, zBax2 was functionally similar to human Bak,24 in that it was unable to interact functionally with zBlp2, the homolog of mammalian Bcl-2. Thus, despite the lack of homology between zebrafish and hum_an Bak, zBax2_ has evolved to display the same interactive selectivity as hBak.
In contrast to zBlp1, zMcl-1a, and zMcl-1b, zBlp2 was only moderately successful in blocking apoptosis induced by ectopic expression of the BH3-only protein zBik. This finding suggests that zBik interacts weakly with zBlp2, providing further evidence for selectivity in the interactions of prosurvival and proapoptotic Bcl-2 family members. Additional selectivity between Bcl-2 family proteins as reported previously6, 33 will be interesting to examine in zebrafish.
We used the zebrafish model to gain further insight into the relative importance of Bcl-2 family members in response to DNA damage induced by _γ_-irradiation. Our results showed that in response to _γ_-irradiation, the apoptosis effector caspase-3 was activated in a _p53_-dependent manner. Overexpression of any of the zebrafish prosurvival Bcl-2 members inhibited irradiation-induced caspase-3 activation. Knockdown of zBax1 also ameliorated this response, confirming that the zebrafish Bcl-2 family regulates the _p53_-dependent apoptotic response to _γ_-irradiation. Furthermore, _γ_-irradiation massively upregulated zPuma transcription through a _p53_-dependent mechanism, whereas only minimally inducing zNoxa. Knockdown of zPuma, but not of zNoxa, abrogated _γ_-irradiation-induced activation of caspase-3. Previous studies have demonstrated the relative importance of Puma versus Noxa in the apoptotic response to _γ_-irradiation of mammalian cells in vitro and within the adult mouse.26 Our data support the conclusion that Puma is the primary initiator of _γ_-irradiation-induced apoptosis in intact, developing vertebrate embryos.
We examined the role of prosurvival Bcl-2 family members during embryonic development. Knockdown of both zMcl-1a and zMcl-1b significantly decreased embryonic viability by 8 h.p.f. In contrast, knockdown of zBlp2 alone or in combination with zMcl-1a or zMcl-1b had no effect on embryonic survival. These data suggest that zMcl-1a and zMcl-1b specifically act as essential gatekeepers during zebrafish embryonic development, protecting embryos from unchecked apoptotic death. Thus, Mcl-1 plays a crucial antiapoptotic role during early embryonic development of the mouse13 as well as the zebrafish, whereas Bcl-2 is dispensable during early embryogenesis in vertebrates. It remains to be determined whether, similar to mammalian Bcl-2, zBlp2 plays a role later in embryogenesis.12, 34
Our results show that zMcl-1a and zMcl-1b function also as gatekeepers against apoptosis induction by stimulation of the extrinsic pathway. In zebrafish embryos, knockdown of zMcl-1a and zMcl-1b substantially increased apoptosis triggered by ectopic expression of the Apo2L/TRAIL homolog zDL1b. Previous work suggests that in human hepatocellular carcinoma-derived cells,32 human cholangiocarcinoma cells,31 and NIH3T3 mouse fibroblasts,30 Mcl-1 is a critical modulator of sensitivity to apoptosis signaling by Apo2L/TRAIL. Hence, endogenous Mcl-1 may be particularly important among prosurvival Bcl-2 family members in controlling apoptosis induction by stimuli that engage both the extrinsic and intrinsic apoptosis pathways.
Several other components of the cell-intrinsic apoptosis pathway have yet to be elucidated in zebrafish. Zebrafish homologs to cytochrome c subunits have been identified.35 Also, a homolog to mammalian caspase-9 has been verified recently (Eimon et al., accompanying manuscript), and potential homologs of Apaf-1, XIAP, and SMAC/Diablo (accession number XP_698996) have been reported14 or predicted, although their functions have not been verified. Our data and previous reports demonstrate that the zebrafish has a functional caspase-3 homolog.36 Future studies will examine if the rest of the intrinsic apoptosis pathway is as functionally conserved as the Bcl-2 gene family in zebrafish and mammals.
Materials and Methods
Zebrafish care
Adult Tubingen long-fin fish were obtained from the Zebrafish International Resource Center. Fish were maintained at 28.5°C according to the standard laboratory practices.37 Embryos were collected ∼30 min postfertilization and maintained in embryo media for further analysis.37 All experiments on live animals were approved by the Genentech Inc. Institutional Animal Care and Use Committee.
Identification of zebrafish Bcl-2 genes
Several zebrafish Bcl-2 genes have been previously reported in the literature.14, 16, 17, 36, 38, 39 zBmf1 and zNoxa homologs were identified by the Strasser group. Novel genes, with the exception of zBik, zPuma, and zBax1, were identified by BLASTing human and mouse protein sequences against the translated zebrafish genome and generating potential coding regions using GenomeScan.40 zBik, zPuma, zBmf2, and zBax2 were identified by Mukhyala et al. (manuscript in preparation). Coding regions were then amplified by RT-PCR with Qiagen OneStep RT-PCR kit according to the manufacturer's directions, and cloned by Topo TA cloning into pCRII (Invitrogen). Multiple RT-PCR products were sequenced to verify the correct coding sequence.
Synteny was established by comparing flanking genes to the zebrafish gene and human homolog via www.ensembl.org, version 39. Genes were designated syntenic if more than a single flanking gene was conserved between zebrafish and human.
Stage-specific and tissue-specific RT-PCR
RNA was isolated from dechorionated wild-type zebrafish embryos at the time points indicated using QiaShredder (Qiagen), followed by Qiagen RNeasy Mini-kit with DNaseI digestion according to the manufacturer's directions. Adult tissues were isolated from an adult female zebrafish. RNA was isolated similarly to embryonic tissue. RT-PCR (Qiagen OneStep) was performed on 50 ng of adult or 100 ng of total embryonic RNA for 30 cycles.
Synthetic mRNA synthesis and microinjection
Zebrafish cDNAs were cloned into the expression vector pCS108 (a gift from R Harland, University of California, Berkeley, CA, USA). Capped synthetic mRNA was generated by Ambion mMessage mMachine, and purified over NucAway spin columns (Ambion Inc.) according to the manufacturer's directions. mRNA was then diluted to the appropriate concentration in 1 × Danieu's solution37+0.2% phenol red.
One- to four-cell stage embryos were injected with a total volume of 4.6 nl using a Nanoliter 2000 (World Precision Instruments) microinjector.
Antisense MO knockdown
MOs were designed around the translational start site of each transcript.41 MOs were obtained from GeneTools LLC, then diluted to 1 mg/ml in 1 × Danieu's solution+0.2% phenol red. A total of either 4.6 or 9.2 ng of MO was injected. In those experiments where a combination of two MOs was used, 4.6 ng of each MO was injected. An additional 4.6 ng of control MO was added to single MO injections when the experiment included comparison with two MO injections, bringing the total MO dose to 9.2 ng for each condition. One- to four-cell stage embryos were injected in a total volume of 4.6 nl via a Nanoliter 2000 (World Precision Instruments) microinjector. MO sequences are listed 5′–3′:
zMcl-1a MO:
5′-GCCTAAAATCCAAACTCAGAGCCAT-3′
zMcl-1b MO:
5′-TGTCGTTGTTTCTTCCAGCGAACAT-3′
zBlp1 MO:
5′-AGGTTGTTGCTCGTTCTCCGATGTC-3′
zBlp2 MO:
5′-GTCATAGCTAATTTCGTTAGCCATG-3′
zBax1 MO:
5′-TGAAAATAAGCGAACTGAAGAAGAC-3′
zBax2 MO:
5′-ATTTTTCGGCTAAAACGTGTATGGG-3′
zBid MO:
5′-GGTCAAAGTTCCTGTTGAAGTCCAT-3′
zBmf1 MO:
5′-ACACATCATCCTCGTCCTCATCCAT-3′
zNoxa MO:
5′-CTTTCTTCGCCATTTCAGCAAGTTT-3′
zPuma MO:
5′-TGCTTTCCATCTCTGGTCGGGCCAT-3′
zBik MO:
5′-CTACAAACAAGGACACAATGGTGGA-3′
p53 MO42:
5′-GCGCCATTGCTTTGCAAGAATTG-3′
Control MO:
5′-CCTCTTACCTCAGTTACAATTTATA-3′
The control MO was obtained from GeneTools LLC.
MOs to proapoptotic Bcl-2 family members were selected based on their ability to inhibit apoptosis induced by ectopic expression of their cognate mRNA. As MO efficacy could not be verified for zBok1, zBok2, zBmf2, or zBad, we did not include these genes in knockdown analyses. MOs to prosurvival Bcl-2 family members were selected based on their ability to abrogate cognate mRNA-mediated rescue from apoptosis triggered by ectopically expressed zNoxa (data not shown).
Antiactive caspase-3 staining
Active caspase-3 was detected as follows: embryos were fixed at 10 h postfertilization (h.p.f.) in 4% paraformaldehyde in phosphate-buffered saline (PBS) for ∼4 h at room temperature or overnight at 4°C, then dehydrated in methanol for a minimum of 2 h. After rehydration, embryos were washed with water, permeabilized in acetone for 7 min at −20°C, and washed again in water. After several washes with PBS+0.5% Tween-20 (PBST), embryos were blocked for 2 h at room temperature in 5% fetal bovine serum, 2 mg/ml bovine serum albumin in PBST. Staining with rabbit antiactive caspase-3 antibodies (Pharmingen #559565) diluted 1 : 500 in block took place overnight at 4°C. Embryos were then washed several times in PBST before incubation with Cy3-conjugated goat anti-rabbit IgG antibodies (Jackson Immunology #111-166-003), also diluted 1 : 500 in block. Embryos were washed again with PBST before visualization using a Leica MZFL3 fluorescence microscope.
Fluorescence was quantified using ImageJ software (http://rsb.info.nih.gov/ij/). Briefly, the threshold was set to eliminate background fluorescence, and each embryo from a single clutch of embryos was analyzed to generate arbitrary fluorescence units.
_γ_-Irradiation
Embryos were _γ_-irradiated at approximately 7 h.p.f. in 1 ml of embryo media with 50 Gy, using a GammaCell 1000 Elite Cesium source (Nordion International Inc.). Embryos were subsequently moved to a tissue culture dish in a greater volume of embryo media and incubated for 3 h at 28.5°C until further analysis.
Quantitative real-time PCR
Taqman analyses were performed using Applied Biosystems 7500 Real-Time PCR System according to the manufacturer's directions. Primer and probe sequences are listed 5′–3′ below:
zGAPDH forward:
5′-TGC GTT CGT CTC TGT AGA TGT-3′
zGAPDH reverse:
5′-GCC TGT GGA GTG ACA CTG A-3′
zGAPDH probe:
5′-TGT GTG TGT GTG TTA GTT TCT TTT GAC AGT ATT TG-3′
zNoxa forward:
5′-CGA ACC TGT GAC AGA AAC TTG-3′
zNoxa reverse:
5′-CTG CGC GCA CTC TAC TAC A-3′
zNoxa probe:
5′-CGG TTT GCT CTT TCT TCG CCA TTT C-3′
zPuma forward:
5′-GAA CAC ACG GGT TAC AAA AGA C-3′
zPuma reverse:
5′-GAA AAT TCC CAG AGT CTG TAA GTG-3′
zPuma probe:
5′-ACG AGT GCA GGC GCT CTC CTT-3′
Accession codes
Accessions
GenBank/EMBL/DDBJ
Abbreviations
BH:
Bcl-2-homology
DL:
death ligand
MO:
morpholino
References
- Hughes P, Bouillet P and Strasser A . Role of Bim and other Bcl-2 family members in autoimmune and degenerative diseases. Curr. Dir. Autoimmun. 2006; 9: 74–94.
CAS PubMed Google Scholar - Strasser A . The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005; 5: 189–200.
Article CAS PubMed Google Scholar - Strasser A, Huang DC and Vaux DL . The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumorigenesis and resistance to chemotherapy. Biochim. Biophys. Acta 1997; 1333: F151–F178.
CAS PubMed Google Scholar - Borner C . The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol. Immunol. 2003; 39: 615–647.
Article CAS PubMed Google Scholar - Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 2001; 8: 705–711.
Article CAS PubMed Google Scholar - Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 2005; 17: 393–403.
Article CAS PubMed Google Scholar - Certo M, Moore Vdel G, Nishino M, Wei G, Korsmeyer S, Armstrong SA et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006; 9: 351–365.
Article CAS PubMed Google Scholar - Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell 2000; 6: 1389–1399.
Article CAS PubMed PubMed Central Google Scholar - Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 1995; 267: 1506–1510.
Article CAS PubMed Google Scholar - Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H and Loh DY . Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc. Natl. Acad. Sci. USA 1994; 91: 3700–3704.
Article CAS PubMed PubMed Central Google Scholar - Nakayama K, Nakayama K, Dustin LB and Loh DY . T–B cell interaction inhibits spontaneous apoptosis of mature lymphocytes in Bcl-2-deficient mice. J. Exp. Med. 1995; 182: 1101–1109.
Article CAS PubMed Google Scholar - Veis DJ, Sorenson CM, Shutter JR and Korsmeyer SJ . Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 1993; 75: 229–240.
Article CAS PubMed Google Scholar - Rinkenberger JL, Horning S, Klocke B, Roth K and Korsmeyer SJ . Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000; 14: 23–27.
CAS PubMed PubMed Central Google Scholar - Inohara N and Nunez G . Genes with homology to mammalian apoptosis regulators identified in zebrafish. Cell Death Differ. 2000; 7: 509–510.
Article CAS PubMed Google Scholar - Coultas L, Huang DC, Adams JM and Strasser A . Pro-apoptotic BH3-only Bcl-2 family members in vertebrate model organisms suitable for genetic experimentation. Cell Death Differ. 2002; 9: 1163–1166.
Article CAS PubMed Google Scholar - Hsieh YC, Chang MS, Chen JY, Yen JJ, Lu IC, Chou CM et al. Cloning of zebrafish BAD, a BH3-only proapoptotic protein, whose overexpression leads to apoptosis in COS-1 cells and zebrafish embryos. Biochem. Biophys. Res. Commun. 2003; 304: 667–675.
Article CAS PubMed Google Scholar - Arnaud E, Ferri KF, Thibaut J, Haftek-Terreau Z, Aouacheria A, Le Guellec D et al. The zebrafish bcl-2 homologue Nrz controls development during somitogenesis and gastrulation via apoptosis-dependent and -independent mechanisms. Cell Death Differ. 2005; 7: 1128–1137.
Google Scholar - Langenau DM, Jette C, Berghmans S, Palomero T, Kanki JP, Kutok JL et al. Suppression of apoptosis by bcl-2 overexpression in lymphoid cells of transgenic zebrafish. Blood 2005; 105: 3278–3285.
Article CAS PubMed Google Scholar - Her GM, Cheng CH, Hong JR, Sundaram GS and Wu JL . Imbalance in liver homeostasis leading to hyperplasia by overexpressing either one of the Bcl-2-related genes, zfBLP1 and zfMcl-1a. Dev. Dyn. 2006; 235: 515–523.
Article CAS PubMed Google Scholar - Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A et al. Zebrafish hox clusters and vertebrate genome evolution. Science 1998; 282: 1711–1714.
Article CAS PubMed Google Scholar - She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J and Rosen N . The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 2005; 8: 287–297.
Article CAS PubMed PubMed Central Google Scholar - Puthalakath H and Strasser A . Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002; 9: 505–512.
Article CAS PubMed Google Scholar - Day CL, Puthalakath H, Skea G, Strasser A, Barsukov I, Lian LY et al. Localization of dynein light chains 1 and 2 and their pro-apoptotic ligands. Biochem. J. 2004; 377: 597–605.
Article CAS PubMed PubMed Central Google Scholar - Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005; 19: 1294–1305.
Article CAS PubMed PubMed Central Google Scholar - Bladen CL, Lam WK, Dynan WS and Kozlowski DJ . DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res. 2005; 33: 3002–3010.
Article CAS PubMed PubMed Central Google Scholar - Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L et al. BH3-only proteins Puma and Bim are rate-limiting for gamma-radiation- and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood 2005; 106: 4131–4138.
Article CAS PubMed PubMed Central Google Scholar - Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–730.
Article CAS PubMed PubMed Central Google Scholar - Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 2003; 4: 321–328.
Article CAS PubMed Google Scholar - Huang DC, Tschopp J and Strasser A . Bcl-2 does not inhibit cell death induced by the physiological Fas ligand: implications for the existence of type I and type II cells. Cell Death Differ. 2000; 7: 754–755.
Article CAS PubMed Google Scholar - Henson ES, Gibson EM, Villanueva J, Bristow NA, Haney N and Gibson SB . Increased expression of Mcl-1 is responsible for the blockage of TRAIL-induced apoptosis mediated by EGF/ErbB1 signaling pathway. J. Cell. Biochem. 2003; 89: 1177–1192.
Article CAS PubMed Google Scholar - Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004; 64: 3517–3524.
Article CAS PubMed Google Scholar - Wirth T, Kuhnel F, Fleischmann-Mundt B, Woller N, Djojosubroto M, Rudolph KL et al. Telomerase-dependent virotherapy overcomes resistance of hepatocellular carcinomas against chemotherapy and tumor necrosis factor-related apoptosis-inducing ligand by elimination of Mcl-1. Cancer Res. 2005; 65: 7393–7402.
Article CAS PubMed Google Scholar - Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 2005; 17: 525–535.
Article CAS PubMed Google Scholar - Bouillet P, Cory S, Zhang LC, Strasser A and Adams JM . Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev. Cell 2001; 1: 645–653.
Article CAS PubMed Google Scholar - Broughton RE, Milam JE and Roe BA . The complete sequence of the zebrafish (Danio rerio) mitochondrial genome and evolutionary patterns in vertebrate mitochondrial DNA. Genome Res. 2001; 11: 1958–1967.
Article CAS PubMed PubMed Central Google Scholar - Yabu T, Kishi S, Okazaki T and Yamashita M . Characterization of zebrafish caspase-3 and induction of apoptosis through ceramide generation in fish fathead minnow tailbud cells and zebrafish embryo. Biochem. J. 2001; 360: 39–47.
Article CAS PubMed PubMed Central Google Scholar - Westerfield M . The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene: University of Oregon Press, 2000.
Google Scholar - Chen MC, Gong HY, Cheng CY, Wang JP, Hong JR and Wu JL . Cloning and characterization of a novel nuclear Bcl-2 family protein, zfMcl-1a, in zebrafish embryo. Biochem. Biophys. Res. Commun. 2000; 279: 725–731.
Article CAS PubMed Google Scholar - Chen MC, Gong HY, Cheng CY, Wang JP, Hong JR and Wu JL . Cloning and characterization of zfBLP1, a Bcl-XL homologue from the zebrafish, Danio rerio. Biochim. Biophys. Acta 2001; 1519: 127–133.
Article CAS PubMed Google Scholar - Yeh R-FL, L P and Burge CB . Computational inference of homologous gene structures in the human genome. Genome Res. 2001; 11: 803–816.
Article CAS PubMed PubMed Central Google Scholar - Nasevicius A and Ekker SC . Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 2000; 26: 216–220.
Article CAS PubMed Google Scholar - Langheinrich U, Hennen E, Stott G and Vacun G . Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr. Biol. 2002; 12: 2023–2028.
Article CAS PubMed Google Scholar
Acknowledgements
We thank Charles Holst, Leon Parker, Weilan Ye, and members of the Ashkenazi lab for informative discussions, Genentech's oligo synthesis and sequencing facilities, and Noel Largaespada and Rigoberto Estrada for zebrafish care.
Author information
Authors and Affiliations
- Department of Molecular Oncology, Genentech Inc., South San Francisco, CA, USA
E Kratz, P M Eimon & A Ashkenazi - Department of Bioinformatics, Genentech Inc., South San Francisco, CA, USA
K Mukhyala & R Hart - Department of Pathology, Genentech Inc., South San Francisco, CA, USA
H Stern & J Zha - The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia
A Strasser
Authors
- E Kratz
You can also search for this author inPubMed Google Scholar - P M Eimon
You can also search for this author inPubMed Google Scholar - K Mukhyala
You can also search for this author inPubMed Google Scholar - H Stern
You can also search for this author inPubMed Google Scholar - J Zha
You can also search for this author inPubMed Google Scholar - A Strasser
You can also search for this author inPubMed Google Scholar - R Hart
You can also search for this author inPubMed Google Scholar - A Ashkenazi
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA Ashkenazi.
Additional information
Edited by G Melino
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Supplementary information
Rights and permissions
About this article
Cite this article
Kratz, E., Eimon, P., Mukhyala, K. et al. Functional characterization of the Bcl-2 gene family in the zebrafish.Cell Death Differ 13, 1631–1640 (2006). https://doi.org/10.1038/sj.cdd.4402016
- Received: 12 June 2006
- Accepted: 26 June 2006
- Published: 04 August 2006
- Issue Date: 01 October 2006
- DOI: https://doi.org/10.1038/sj.cdd.4402016