Catechol-O-Methyltransferase (COMT) Val158Met Genotype is Associated with BOLD Response as a Function of Task Characteristic (original) (raw)
INTRODUCTION
Behavioral genetic studies have demonstrated that there is a significant genetic contribution to most aspects of cognition, behavior, and its disorders (McGuffin et al, 2001). Recent studies have begun to delineate specific molecular genetic causes and the neural mechanisms through which these genes may act. In human subjects, this research has progressed rapidly through the combination of genotyping and in vivo brain imaging methods, such as functional magnetic resonance imaging (fMRI) (Hariri and Weinberger, 2003; Heinz and Smolka, 2006; Meyer-Lindenberg and Weinberger, 2006; Mitterschiffthaler et al, 2006).
The present study addresses associations with brain function of the catechol-_O_-methyltransferase (COMT) gene, a widely studied candidate gene for cognition, brain function, and psychiatric disorder (Craddock et al, 2006). The COMT enzyme is involved in the catabolism of the catecholamine neurotransmitters dopamine, adrenalin, and noradrenalin. COMT is widely expressed in human brain tissue, but plays a major role in dopamine breakdown in frontal cortex (Hong et al, 1998; Lloyd et al, 1975). The COMT gene (located in 22q11.1–q11.2) contains a G to A missense mutation, resulting in a substitution of methionine (met) for valine (val) at codon 158 of the membrane-bound isoform of the protein (reference sequence identification code rs4680). This allelic variation (val158 met) is a functional polymorphism: the met158 allele has about one-third to one-fourth of the activity of the val158 allele, resulting in less efficient catecholamine catabolism (Lachman et al, 1996; Lotta et al, 1995; Weinshilboum et al, 1999).
Some human studies have shown that the val158 allele is associated with worse performance in executive function and working memory (Egan et al, 2001; Joober et al, 2002), although the overall effect may be small (Flint and Munafo, 2007) and may be seen only in healthy but not schizophrenic individuals (Barnett et al, 2007). The val158 allele has also been shown to be associated with inefficient (ie increased) frontal activation (Blasi et al, 2005; Egan et al, 2001) as well as greater signal-to-noise ratio (Winterer et al, 2006).
Bilder et al (2004) recently argued that the met158 allele is linked to increased tonic and reduced phasic dopamine levels in subcortical regions but increased dopamine levels in cortex. The val158 allele is thought to show the opposite pattern, that is, increased phasic and decreased tonic dopamine transmission subcortically and decreased cortical dopamine concentrations. These effects on dopamine transmission are thought to be reflected at behavioral and cognitive levels, with high tonic dopamine activity (met158) relating to better performance on tasks measuring cognitive stability, while higher phasic dopamine turnover (val158) may relate to better performance on tasks of cognitive plasticity (Nolan et al, 2004). Measures of stability are defined as those involving the representation of task set and continuous, sustained attention, whereas measures of plasticity are thought to include the adaptive responding to shifts in the current plans, dealing with deviations and errors, and mediating reward incentives (Bilder et al, 2004).
The present study employed prosaccade and antisaccade tasks to test the associations of the COMT val158met polymorphism with measures of brain function in healthy humans. A prosaccade is a rapid eye movement that coincides with a shift of attention (Deubel and Schneider, 1996; Hoffman and Subramaniam, 1995). The antisaccade task is a measure of the ability to suppress an unwanted reflexive response (a saccade toward the peripheral target) in favor of a volitional response (an antisaccade in the opposite direction) (see for review Hutton and Ettinger, 2006). While both tasks involve representation of task set and sustained attention, antisaccades additionally involve the inhibition of inappropriate responses and the monitoring and correction of errors. Prosaccades may thus best be understood in terms of stability, whereas antisaccades may represent a measure of plasticity. We adopted a previously employed approach (Flint and Munafo, 2007; Meyer-Lindenberg et al, 2005; Winterer et al, 2006) and compared val158 allele carriers (ie val158 homozygotes and val158met heterozygotes) to val158 noncarriers (ie met158 homozygotes).
While the performance of saccadic tasks engages well-known dorsal fronto-parieto-striato-thalamic networks, gene effects may also be found in deactivation areas, that is, brain regions that show lower blood oxygenation level-dependent (BOLD) response during an active task relative to a low-demand control condition (Raichle et al, 2001). It has been shown that a network of medial frontal (including dorsomedial and ventromedial prefrontal cortex) and medial (posterior cingulate and precuneus) as well as lateral parietal areas is more strongly activated during rest (Mazoyer et al, 2001) or low-demand baseline tasks (Gilbert et al, 2006) relative to complex, higher-level tasks. While the precise function of this deactivation network is unclear, it has been suggested that it mediates stimulus-independent thought processes such as mind wandering (Mason et al, 2007) or stimulus-oriented processes such as enhanced attention to the external environment (Gilbert et al, 2007).
On the basis of Bilder's hypothesis, it may be expected that val158 carriers would show better performance on antisaccades than met158 homozygotes, which may be manifested at a neural level as either less (more efficient) activation in task-related areas or larger deactivations of fronto-parietal deactivation areas; the inverse pattern was expected for prosaccades. These assumptions are based on fMRI evidence showing that greater deactivations are associated with better performance on a trial-by-trial basis (Polli et al, 2005; Weissman et al, 2006) as well as in analyses of interindividual differences (Harrison et al, 2007); conversely, it has been found that lesser (ie more efficient) BOLD response in areas activated by a task is associated with better performance (Blasi et al, 2005; Rypma and D’Esposito, 1999).
MATERIALS AND METHODS
Participants
We recruited right-handed (Oldfield, 1971) healthy volunteers from the university staff and students. All participants were Caucasian and free of current or past psychiatric illness and of a family history of psychosis in first-degree relatives. Parental socioeconomic status (SES) was measured separately for father and mother on a 4-point scale (The Stationary Office, 2000) of standard occupational classifications (1 being the lowest, 4 being the highest). All participants provided written informed consent and the study had ethical approval.
DNA Extraction and Genotyping
Deoxyribonucleic acid (DNA) was obtained from venal blood or buccal swabs using established procedures (Freeman et al, 2003). COMT val158met (rs4680) genotype was determined by a TaqMan Drug Metabolising Genotyping assay (Applied Biosystems, Assay ID C_25746809_50) following the manufacturer's instructions. Endpoint analysis was performed on an ABI7900 DNA analyzer and genotypes called with the SDS package with a probability greater than 95%. To improve genotyping reliability, many samples of similar DNA quality and concentration were genotyped at the same time.
fMRI Data Acquisition
Scanning was performed on a 1.5 T GE Signa Advantage MRI scanner. Participants wore headphones to reduce the impact of scanner noise. Head movements were minimized using foam padding and a forehead band. A localizer scan for placing the volume of interest and a high-resolution structural scan for image co-registration were first acquired.
T2-weighted MR echo-planar images of the whole brain showing the BOLD response were collected. A quadrature headcoil was used for radiofrequency transmission and reception. Images were aligned parallel to the intercommissural plane (AC–PC line). Image parameters were as follows: repetition time (TR)=2 s, echo time (TE)=40 ms, field of view (FOV)=24 cm, flip angle=80° and in-plane resolution=3.75 × 3.75 mm, yielding 22 axial slices of slice thickness 5 mm with 0.5 mm inter-slice gap. A total of 240 images were acquired in one continuous run with a total scanning time per subject of 8 min. Four 2-s scans of dummy data acquisition were performed before each run to allow for the establishment of steady-state longitudinal magnetization; these scans were not used in the analysis.
Task Design and Stimuli
A block-design was employed, consisting of five blocks of antisaccades, five blocks of prosaccades, and five blocks of fixation presented in the same quasi-random order for each participant. Each block lasted 30 s. There were 10 trials in each saccade block. Each trial consisted of a central target and a peripheral target. Target presentations were quasi-random (1100–1900 ms) and were timed such that each trial lasted 3 s. In antisaccade trials, the central target was red, and in prosaccade trials, it was green following previous procedures (eg Connolly et al, 2002). The peripheral target (±8°) was always black and the background was gray. Equal numbers of right and left targets were used in each block. Participants were instructed to look at the mirror image location of the peripheral target on antisaccade trials and to look at the peripheral target on prosaccade trials. Fixation blocks consisted of a 30 s black central target. On fixation trials, participants were instructed to simply keep their eyes on the central target. There was a 2 s instruction screen before each block, reminding participants of the task (antisaccade: ‘Look Away’; prosaccade: ‘Follow the Dot’; fixation: ‘Center’). All participants were practiced on the task prior to the fMRI scan.
Oculomotor Data Acquisition and Analysis
Horizontal movements of the left eye were recorded using an MRI-compatible infrared oculographic limbus tracker (MR-Eyetracker, CRS Ltd, Rochester, UK). The MR-Eyetracker uses fiber optic cables for guiding infrared light between the hardware in the control room and an eyepiece fixed to the head coil and placed beneath the participant's left eye. The system has a minimum spatial resolution of 0.2° and a horizontal range of ±20°. Signals were digitized using 12-bit analog-to-digital converter (Data Translation DT9802) and sampled at 500 Hz. A three-point calibration (±8°, 0°) was performed before the scan.
Saccades were identified using Eyemap (AMTech GmbH) using minimum latency (100 ms) and amplitude (1°) criteria. Prosaccade and antisaccade latencies were calculated. Additionally, the percentage of reflexive errors on the antisaccade task (saccades to the peripheral target) and the percentage of corrections (antisaccade following a reflexive error) were calculated.
fMRI Data Analysis
Analysis was carried out using Statistical Parametric Mapping 2 (SPM2) software (http://www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 6. Preprocessing consisted of three steps. First, motion correction was carried out by realigning each subject's images along the first image in the time series. Second, images were transformed into standard space using affine registration and nonlinear transformations and the Montreal Neurological Institute template in SPM2. Third, images were spatially smoothed with an 8 mm Gaussian full-width at half-maximum (FWHM) filter.
Data were analyzed with a general linear model in SPM2, involving two steps. First, single-subject maps were obtained for prosaccade>fixation and antisaccade>prosaccade. Fixation was used as low-level comparison condition for prosaccades to study brain activation in relation to saccadic eye movements, as carried out previously (McDowell et al, 2002; Sweeney et al, 1996). The prosaccade task was used as a comparison (or baseline) task for the antisaccade task (as described previously, eg McDowell et al, 2002; O’Driscoll et al, 1995; Sweeney et al, 1996), as in this part of the experiment, we aimed to study brain activation specific to the cognitive components involving antisaccade task performance. As described before (Hutton and Ettinger, 2006; Reuter and Kathmann, 2004), these processes include response inhibition, complex sensorimotor transformations, and volitional (as opposed to reflexive) response generation. Prosaccades share with antisaccades the recruitment of basic saccadic processes but lack these complex cognitive demands (Sweeney et al, 1996). Additionally, as argued elsewhere (Gilbert et al, 2006), simple reflexive motor tasks (such as prosaccades) can be considered useful low-demand baseline tasks in fMRI experiments and represent appropriate comparison conditions for more complex tasks.
Single subject maps were then combined at the group level in a random effects analysis to produce statistical parametric maps (SPMs) of group activation. A one-sample _t_-test was carried out to investigate activation during prosaccade>fixation and antisaccade>prosaccade. The threshold for significance was set at p<0.05 (corrected cluster level).
To investigate effects of val158met genotype on BOLD response, val158 homozygotes and val158met heterozygotes were combined into a group of val158 allele carriers and compared to val158 allele noncarriers. This was done in order to obtain sufficient group sizes for analysis of between-group effects as implemented previously (Flint and Munafo, 2007; Meyer-Lindenberg et al, 2005; Winterer et al, 2006). Two-sample _t_-tests, unbiased without anatomical restraints, were carried out to compare BOLD response between val158 carriers and val158 noncarriers. The threshold for significance was set at p<0.05 (corrected) at the cluster level with a height threshold of p<0.001 at the voxel level. We also applied small volume corrections (SVC; consisting of a sphere with radius=10 mm) to clusters differing between val158 carriers and noncarriers at p<0.05 (uncorrected).
MNI coordinates of identified areas given in SPM2 were converted to Talairach coordinates (Talairach and Tournoux, 1988) using a nonlinear transformation algorithm (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach; Brett et al, 2002). Macroanatomical localization of the peak voxel in each cluster and corresponding Brodmann area (BA) was identified on the basis of the stereotaxic atlas by Talairach and Tournoux (1988). The functional labels frontal and supplementary eye fields were chosen on the basis of previous literature (Grosbras et al, 2005).
RESULTS
Genotype, Demographic, and Oculomotor Variables
Thirty-six participants completed the study. There were 6 val158 homozygotes, 18 val158met heterozygotes, and 12 met158 homozygotes. The genotype distribution did not deviate significantly from Hardy–Weinberg equilibrium (_p_=0.98). Descriptive statistics of demographic and oculomotor variables are summarized in Table 1. Val158 carriers (_N_=24) did not significantly differ from val158 noncarriers (_N_=12) in demographic variables or oculomotor performance.
Table 1 Demographic and Oculomotor Variables by Group
Activations and Deactivations during Prosaccades and Antisaccades
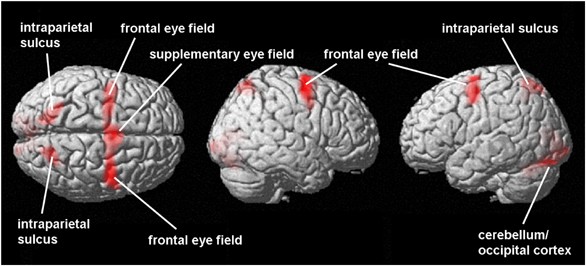
Table 2 and Figure 1 show the network of brain activation during prosaccades compared to fixation (prosaccade>fixation). This contrast shows activation bilaterally in the frontal eye fields, the supplementary eye field (one cluster in the superior frontal gyri of both hemispheres), bilaterally in the intraparietal sulcus (including the parietal eye fields), and in occipital cortex. The cluster in the occipital cortex extended bilaterally into the cerebellum. This network replicates the well-established cortical areas supporting prosaccades (Grosbras et al, 2005).
Table 2 BOLD Response during Prosaccades in the Combined Group
Figure 1
BOLD response during prosaccades in the combined group. The figure shows activations (in red) during prosaccades (relative to fixation) in the combined group (_N_=36). Significance is thresholded at p<0.05 (corrected cluster level) (_N_=36).
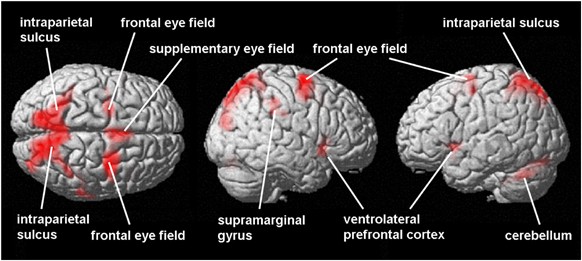
Table 3 and Figure 2 show the network of brain activation during antisaccades compared to prosaccades (antisaccade>prosaccade). This activation network overlaps considerably with that seen for prosaccades, including frontal eye fields, supplementary eye field, intraparietal sulcus, and occipital cortex. Additionally, there is activation bilaterally in the ventrolateral prefrontal cortex. These areas are in agreement with previous neuroimaging studies of antisaccade eye movements (Hutton and Ettinger, 2006; Munoz and Everling, 2004).
Table 3 BOLD Response during Antisaccades in the Combined Group
Figure 2
BOLD response during antisaccades in the combined group. The figure shows activations (in red) during antisaccades (relative to prosaccades) in the combined group (_N_=36). Significance is thresholded at p<0.05 (corrected cluster level).
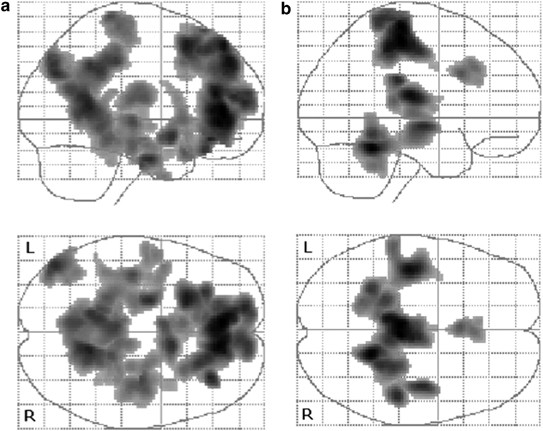
Areas of deactivation during prosaccades (fixation>prosaccade) were found in a distributed network of five extensive clusters (Figure 3): (1) posterior cingulate and precuneus; (2) right middle temporal gyrus, inferior parietal lobule, and cerebellum; (3) left postcentral gyrus and superior temporal gyrus; (4) left fusiform gyrus extending into the left cerebellum; and (5) anterior cingulate. These areas lie within previously described networks showing deactivations during the task relative to baseline condition (Gusnard et al, 2001; Mazoyer et al, 2001; McKiernan et al, 2003) and overlap with a previous study of deactivations during prosaccades relative to fixation (Sweeney et al, 1996).
Figure 3
Deactivations during pro- and antisaccades in the combined group. The figure shows clusters of deactivation (shaded in gray) in the combined group (_N_=36). (a) Deactivations during antisaccades (relative to prosaccades). (b) Deactivations during prosaccades (relative to fixation). Significance is thresholded at p<0.05 (corrected cluster level).
Areas of deactivation during antisaccades (prosaccade>antisaccade) were observed in three large clusters (Figure 3): (1) in an extended bilateral fronto-parieto-temporal region including medial frontal gyrus, anterior cingulate, superior frontal gyrus, orbitofrontal cortex, the medial aspects of middle frontal and inferior frontal gyrus, posterior cingulate gyrus, precuneus, and middle temporal gyrus; (2) in the left angular gyrus; and (3) in the right postcentral gyrus and superior temporal gyrus. This network overlaps with previous findings of deactivations in ventromedial and orbital frontal areas during antisaccades relative to prosaccades (Sweeney et al, 1996) and deactivations in precuneus, posterior cingulate, and angular gyrus during cue-instructed, volitional saccades relative to prosaccades (Mort et al, 2003). The network is also similar to areas reported during a simple reaction time baseline task relative to more complex tasks by Gilbert et al (2006).
Effects of COMT Val158Met Genotype on BOLD during Prosaccades
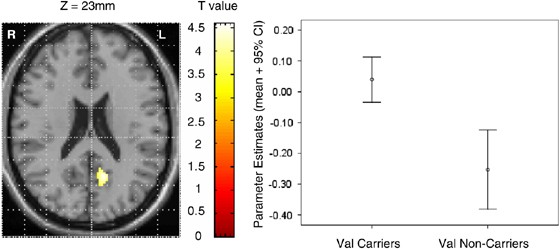
The two-sample _t_-test for the comparison of val158 carriers and noncarriers showed one discrete cluster (cluster size=167 voxels). Val158 carriers showed greater activation levels than noncarriers in the medial parietal cortex (Figure 4) in BA23/31, including precuneus and posterior cingulate (_x_=−12, _y_=−57, _z_=23). The difference was significant at the cluster level (_p_=0.02 uncorrected; _p_=0.002 corrected with SVC). The mean parameter estimate for the combined group was −0.06 (SD=0.23) (Figures 4 and 5).
Figure 4
Differences in BOLD response during prosaccades between Val158 carriers>Val158 noncarriers. The axial brain image on the left displays the difference in activation between val158 carriers (_N_=24) and val158 noncarriers (_N_=12) during prosaccades imposed on a single subject's T1-weighted scan. The graph on the right shows the parameter estimates (mean+95% confidence intervals) for the peak voxel in the cluster (_x_=−12, _y_=−57, _z_=23). The parameter estimates equate to percent change in the global mean BOLD signal.
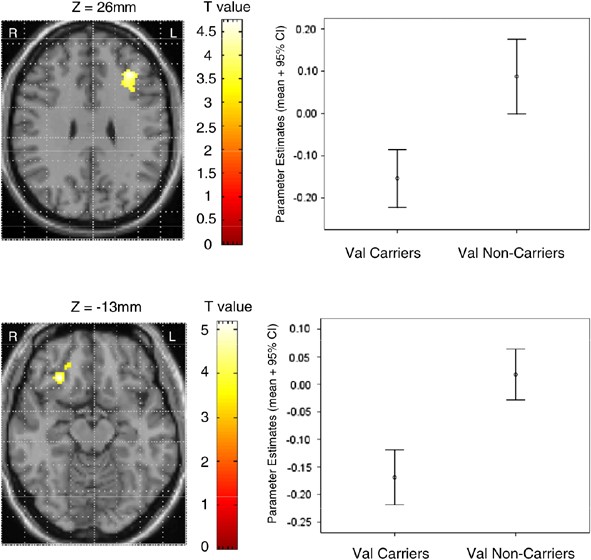
Figure 5
Differences in BOLD response during antisaccades between Val158 carriers and Val158 noncarriers. The axial brain images on the left display the differences in activation between val158 carriers (_N_=24) and val158 noncarriers (_N_=12) during antisaccades imposed on a single subject's T1-weighted scan. The charts on the right show the parameter estimates (mean+95% confidence intervals) for the peak voxel in each cluster (upper panel: _x_=−32, _y_=30, _z_=26; lower panel: _x_=30, _y_=28, _z_=−13). The parameter estimates equate to percent change in the global mean BOLD signal.
Further inspection of the data of the subgroups within the val158 allele carriers (ie val158 homozygotes vs val158met heterozygotes) showed that the two groups of val158 carriers showed similar mean parameter estimates (val158 homozygotes: 0.002; val158met heterozygotes: 0.05) when compared to met158 homozygotes. Met158 homozygotes, however, differed clearly from the two val158 carrier groups (Figure 4). Additionally, given the smaller sample size of the val158 homozygotes, the 95% confidence intervals for β coefficients in this group were larger than in the val158met heterozygotes (val158 homozygotes: −0.23 to 0.24; val158met heterozygotes: 0.05 to −0.03); 95% confidence intervals in the val158 group were also larger than in the met158 homozygotes (see Figures 4 and 5). These data support the strategy of combining val158 homozygotes and val158met heterozygotes and comparing these to met158 homozygotes (Flint and Munafo, 2007; Winterer et al, 2006).
Effects of COMT Val158Met Genotype on BOLD during Antisaccades
The two-sample _t_-tests for the comparison of val158 carriers and val158 noncarriers showed two clusters (Figure 5). In both clusters, val158 noncarriers showed greater activation levels than val158 carriers. The clusters focused on the medial aspect of the ventral prefrontal cortex in BA47 (_x_=30, _y_=28, _z_=−13; cluster size=146 voxels) and the medial aspect of the dorsal prefrontal cortex in BA9 (_x_=−32, _y_=30, _z_=26; cluster size=215 voxels). The differences were significant at the cluster level for BA47 (_p_=0.03 uncorrected; _p_=0.003 corrected with SVC) and BA9 (_p_=0.01 uncorrected; _p_=0.002 corrected with SVC). The mean parameter estimates for these areas in the combined group were −0.11 (SD=0.14) for BA47 and −0.07 (SD=0.19) for BA9.
Further inspection of the data of the subgroups within the val158 allele carriers showed that the two subgroups (val158 homozygotes and val158met heterozygotes) showed similar mean parameter estimates for BA47 (val158 homozygotes: −0.14; val158met heterozygotes: −0.19) and BA9 (val158 homozygotes: −0.09; val158met heterozygotes: −0.17) when compared to met158 homozygotes. Met158 homozygotes, however, differed clearly from the two val158 carrier groups (Figure 5). Additionally, given the smaller sample size of the val158 homozygotes, the 95% confidence intervals for β coefficients in this group were larger than in the val158met heterozygotes for BA47 (val158 homozygotes: −0.24 to −0.04; val158met heterozygotes: −0.24 to −0.12) and BA9 (val158 homozygotes: −0.24 to 0.05; val158met heterozygotes: −0.26 to −0.09); 95% confidence intervals in the val158 homozygote group were also larger than in the met158 homozygotes (Figure 5). As for prosaccades, these data agree with the strategy of combining val158 homozygotes and val158met heterozygotes and comparing these to met158 homozygotes.
Interaction between Area, Task, and Group
Given that different, regionally specific group differences emerged for the two different contrasts, we aimed to explore whether this pattern could be confirmed by a significant group-by-task-by-area interaction. Parameter estimates from the peak voxel within each cluster were entered into repeated measures ANOVA with group (val158 carriers, val158 noncarriers) as between-group factor and task (antisaccade, prosaccade) and area (BA9, BA47, BA23/31) as within-subjects factor. As predicted, there was a significant interaction (F (2,68)=23.71, p<0.001). This interaction indicated that val158 carriers showed lower activations than val158 noncarriers in frontal areas only during antisaccades, while val158 noncarriers showed lower activations than val158 noncarriers in BA23/31 only during prosaccades.
DISCUSSION
In this study, we investigated the association between COMT val158met genotype and brain function. We found that genotype is associated with BOLD response as a function of task characteristic: val158 allele noncarriers showed lower BOLD response than val158 carriers in posterior cingulate/precuneus during prosaccades, whereas val158 allele carriers showed lower BOLD response than noncarriers in dorsomedial and ventromedial prefrontal areas during antisaccades. This pattern of findings suggests that the association between COMT val158met genotype and BOLD response depends on the type of behavioral probe used to elicit brain function.
Bilder et al (2004) postulated that the met158 allele should be associated with better performance on measures of stability, whereas the val158 allele should be associated with better performance on tasks involving plasticity or flexibility. Applied to the present tasks, this theory predicts that val158 noncarriers should show superior prosaccade performance, whereas val158 carriers should show superior antisaccade performance. As outlined above, both prosaccade and antisaccade tasks involve elements of cognitive stability, such as the representation of task set and sustained attention. Importantly, antisaccades (but not prosaccades) additionally require features of plasticity tasks, such as inhibition of inappropriate responses and monitoring and correction of errors. Directional errors occur on a substantial proportion of trials on antisaccade tasks (Hutton and Ettinger, 2006) but very rarely on prosaccade trials, with the vast majority of subjects not committing any errors. In this and previous studies, directional errors in the antisaccade task are corrected in the vast majority of trials, requiring error monitoring and correction processes (Nieuwenhuis et al, 2001; Polli et al, 2005). Finally, antisaccade performance is sensitive to monetary reward incentives (Duka and Lupp, 1997; Jazbec et al, 2005). Therefore, for the purpose of the present discussion, prosaccade tasks may be considered a measure of stability, whereas antisaccade tasks may represent a measure of plasticity.
In the present study, there were no gene effects on oculomotor performance (although val158 carriers showed nonsignificantly better scores on antisaccade error and correction rate), likely in part due to the relatively small number of subjects in this sample and the small magnitude of gene effects on behavior. Our findings are thus compatible with the notions (1) that single-gene effects on behavior require large samples to detect and (2) that functional neuroimaging endophenotypes may be more sensitive at detecting gene effects than behavioral performance measures (Hariri and Weinberger, 2003). Formal power calculation indicated that for the key performance measure of antisaccade errors, 484 subjects per group are needed to achieve 80% power of detecting a significant (p<0.05) effect.
The present data may provide a neurophysiological explanation of the hypothesized (Bilder et al, 2004) and observed (Nolan et al, 2004) COMT val158met effects on neurocognitive performance. It can be inferred on the basis of our data that one possible reason for the differential gene effects hypothesized in Bilder et al (2004) is the ability to appropriately deactivate brain areas in the medial fronto-parietal network that show greater activations during low-level control tasks compared to active tasks (Gusnard et al, 2001; Mazoyer et al, 2001; McKiernan et al, 2003; Raichle et al, 2001). Our data suggest that val158 carriers appropriately deactivate these areas during antisaccades (a measure of cognitive flexibility), whereas val158 noncarriers appropriately deactivate these areas during prosaccades (a measure of stability). Our interpretation is compatible with previous observations of relationships between better performance and greater deactivations both at the level of interindividual (Harrison et al, 2007) and trial-by-trial differences (Polli et al, 2005; Weissman et al, 2006) and in pharmacological studies (Hahn et al, 2007). Additionally, it has been observed that a number of neuropsychiatric patient groups, such as people with autism (Kennedy et al, 2006), fragile X syndrome (Menon et al, 2004), Parkinson's disease (Tinaz et al, 2006), or dementia (Sauer et al, 2006), fail to show (or show lower levels of) the typical pattern of deactivations seen in healthy, normal-performing controls.
While little is known about the role of dopamine in the brain deactivation patterns detected in fMRI, it has been found that individual differences in extraversion, a personality trait mediated by dopamine turnover (Rammsayer, 2004), are associated with levels of activation and deactivation in medial fronto-parietal brain areas (Kumari et al, 2004). It is also of interest to acknowledge that in the present study, there were no differences in task-activated brain areas (such as those displayed in Figures 1 and 2). It is unclear why we did not find differences in this network, but our findings suggest that COMT val158met effects during the particular tasks selected here may be stronger at the level of deactivation than activation areas. Larger samples are needed to address this issue more conclusively.
In this context, it is important to note that the frontal areas found to differ between groups in this study do not correspond to dorsolateral and ventrolateral prefrontal cortices seen during antisaccade activation in this and previous studies (McDowell et al, 2002; Sweeney et al, 1996); instead, they are more medial and correspond better to typical deactivation areas (Gusnard et al, 2001; Mazoyer et al, 2001; McKiernan et al, 2003). While the two frontal clusters observed here are not mid-line structures, they are close to peaks described in a meta-analysis (see Table 4 in Mazoyer et al, 2001) and shown in an illustrative overview of deactivation areas (see Figure 1 in McKiernan et al, 2003). As described in Results, the overall group means at the areas of difference between val158 carriers and noncarriers were negative. This finding confirms that in the combined group, the areas were deactivated during active task compared to control conditions; however, the overall level of deactivation was weak and the genetic findings suggest that there was significant between-subject variance explained by COMT val158met genotype.
The task-related areas of activation are in good concordance with previous block-design functional neuroimaging studies of reflexive saccades and antisaccades in healthy humans (McDowell et al, 2002; O’Driscoll et al, 1995; Sweeney et al, 1996). Prosaccades and antisaccades share recruitment of dorsal frontal and parietal oculomotor areas. Additionally, antisaccades have been shown to involve ventrolateral prefrontal cortex (Tu et al, 2006; Walker et al, 1998) and supramarginal gyrus (Chikazoe et al, 2007; Matsuda et al, 2004). Activation in the dorsolateral prefrontal cortice, an area of overall importance in antisaccade performance (Dyckman et al, 2007; Ploner et al, 2005), was seen in the present study but did not survive correction for multiple comparisons.
The present study may have implications for psychiatric genetics, in particular with regard to schizophrenia. The COMT val158met polymorphism has been implicated in a number of neuropsychiatric conditions (Tunbridge et al, 2006). It has been widely studied in relation to schizophrenia, although the overall association may be very small (Munafo et al, 2005). Given that associations between specific candidate genes and the schizophrenia phenotype appear to be of small effect size, it has been suggested that the study of intermediate phenotypes, or endophenotypes, may be advantageous (Braff et al, 2007; Gottesman and Gould, 2003). Endophenotypes are thought to be a more proximal and homogeneous measure of a gene effect than the complex behavioral (or disease) phenotype and may therefore be used profitably in molecular genetic designs, such as linkage and association studies (Braff et al, 2007; Gottesman and Gould, 2003). While a large number of previous studies have employed cognitive and neuropsychological endophenotypes, it has recently been suggested that the brain functional response may be a more proximal and neurobiologically plausible intermediate phenotype (Meyer-Lindenberg and Weinberger, 2006).
The present findings may be viewed within this framework, specifically with regard to intermediate phenotypes for schizophrenia. Hariri and Weinberger (2003) outlined three principles that should be considered to facilitate detection of meaningful gene effects on brain function using functional imaging. First, the polymorphism under study should be a well-defined, functional polymorphism with candidate gene status for a certain behavior or disorder. Second, groups (eg allele carriers vs noncarriers) should be matched on important demographic variables to avoid masking true gene effects on brain function or producing spurious ones. Third, the task used to elicit brain function should be a well-characterized paradigm known to produce robust activation in relevant brain regions.
The present study clearly fulfills these criteria: first, COMT val158met is one of the most widely studied, functional polymorphisms in psychiatry and cognitive neuroscience, in particular with regard to schizophrenia and frontal lobe function (for reviews on the val158met polymorphism, see Barnett et al, 2007; Bilder et al, 2004; Flint and Munafo, 2007; Heinz and Smolka, 2006; Munafo et al, 2005; Savitz et al, 2006; Tunbridge et al, 2006; Winterer and Goldman, 2003). Second, val158 carriers and noncarriers in this study did not significantly differ in age, gender, ethnicity, handedness, level of education, or parental socioeconomic status. Third, the antisaccade task is a paradigm that has been studied in detail with regard to its status as a candidate endophenotype for schizophrenia. Antisaccade performance is a temporally stable trait marker with significant impairments in schizophrenia (Calkins et al, 2003; Curtis et al, 2001; Ettinger et al, 2003; Gooding et al, 2005). The task recruits well-replicated fronto-parieto-striatal networks (Müri et al, 1998; O’Driscoll et al, 1995; Sweeney et al, 1996), which are abnormal in schizophrenia patients (McDowell et al, 2002; Raemaekers et al, 2002) and their relatives (Raemaekers et al, 2006). Antisaccade impairments are observed in people at risk for schizophrenia, for example, the unaffected biological relatives of schizophrenia patients (Calkins et al, 2004; Clementz et al, 1994; Curtis et al, 2001; Ettinger et al, 2006), individuals at ultra-high risk for schizophrenia (Nieman et al, 2007), and people with heightened scores of schizotypal traits (Ettinger et al, 2005; O’Driscoll et al, 1998; Smyrnis et al, 2003).
Given this evidence of antisaccade impairments across the schizophrenia spectrum, it would be of interest to study the relationship between COMT val158met genotype and BOLD response in people with schizophrenia as well as genetic high-risk populations, such as biological relatives of schizophrenia patients or people with schizotypal personality traits. In this context, it is important to note, however, that the present study of healthy individuals has certain methodological advantages over patient studies. Healthy controls do not display confounds such as pharmacological treatment, acute symptoms, and factors secondary to the disease that might affect performance and/or brain function. Therefore, the study of healthy individuals provides an important model within which to characterize gene effects on brain function.
LIMITATIONS
A number of limitations of the present study should be acknowledged. First, while gender distribution did not significantly differ between genotype groups, it should be noted that two-thirds of val158 allele carriers were male compared to only one-third of val158 allele noncarriers. The sample sizes prohibited a more detailed analysis of gender or gender-by-genotype effects on brain function; this issue should be considered in future studies.
Second, the attribution of prosaccades and antisaccades to the domains of cognitive stability and plasticity, respectively, may, to some extent, be confounded by the level of task difficulty. Future research is needed to investigate genetic and brain functional correlates of stability and plasticity measures matched for the level of task difficulty.
Third, the sample size of the present study was small compared to large-scale investigations of genes with small effects (Craddock et al, 2006; Wang et al, 2005) and the results regarding the COMT effects were observed at the uncorrected level. While the sample size was comparable to some previous fMRI studies of gene effects (Blasi et al, 2005; Winterer et al, 2006), future research will be needed to test whether the gene–brain associations observed here are reproducible.
CONCLUSIONS
We observed associations between COMT val158met genotype and BOLD response during prosaccade and antisaccade task performance in the absence of a gene effect at the behavioral level. The findings are compatible with the notion that the val158 allele is associated with improved brain plasticity performance, while the met158 allele is associated with improved brain stability performance (Bilder et al, 2004). Future research is required to replicate the present findings using larger samples in order to provide further evidence of validity. The present design may also be extended to schizophrenia patients and those at risk for the illness.
References
- Barnett JH, Jones PB, Robbins TW, Muller U (2007). Effects of the catechol-_O_-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry 12: 502–509.
Article CAS PubMed Google Scholar - Bilder RM, Volavka J, Lachman HM, Grace AA (2004). The catechol-_O_-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29: 1943–1961.
Article CAS PubMed Google Scholar - Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S et al (2005). Effect of catechol-_O_-methyltransferase val158met genotype on attentional control. J Neurosci 25: 5038–5045.
Article CAS PubMed PubMed Central Google Scholar - Braff DL, Freedman R, Schork NJ, Gottesman II (2007). Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull 33: 21–32.
Article PubMed Google Scholar - Brett M, Johnsrude IS, Owen AM (2002). The problem of functional localization in the human brain. Nat Rev Neurosci 3: 243–249.
Article CAS PubMed Google Scholar - Calkins ME, Curtis CE, Iacono WG, Grove WM (2004). Antisaccade performance is impaired in medically and psychiatrically healthy biological relatives of schizophrenia patients. Schizophr Res 71: 167–178.
Article PubMed Google Scholar - Calkins ME, Iacono WG, Curtis CE (2003). Smooth pursuit and antisaccade performance evidence trait stability in schizophrenia patients and their relatives. Int J Psychophysiol 49: 139–146.
Article PubMed Google Scholar - Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y (2007). Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci 19: 69–80.
Article PubMed Google Scholar - Clementz BA, McDowell JE, Zisook S (1994). Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol 103: 277–287.
Article CAS PubMed Google Scholar - Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences. Lawrence Earlbaum Associates, Inc.: Hillsdale, NJ.
Google Scholar - Connolly JD, Goodale MA, Menon RS, Munoz DP (2002). Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci 5: 1345–1352.
Article CAS PubMed Google Scholar - Craddock N, Owen MJ, O’Donovan MC (2006). The catechol-_O_-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol Psychiatry 11: 446–458.
Article CAS PubMed Google Scholar - Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG (2001). Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. Am J Psychiatry 158: 100–106.
Article CAS PubMed Google Scholar - Deubel H, Schneider WX (1996). Saccade target selection and object recognition: evidence for a common attentional mechanism. Vis Res 36: 1827–1837.
Article CAS PubMed Google Scholar - Duka T, Lupp A (1997). The effects of incentive on antisaccades: is a dopaminergic mechanism involved? Behav Pharmacol 8: 373–382.
Article CAS PubMed Google Scholar - Dyckman KA, Camchong J, Clementz BA, McDowell JE (2007). An effect of context on saccade-related behavior and brain activity. NeuroImage 36: 774–784.
Article PubMed Google Scholar - Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE et al (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917–6922.
Article CAS PubMed PubMed Central Google Scholar - Ettinger U, Kumari V, Crawford TJ, Davis RE, Sharma T, Corr PJ (2003). Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology 40: 620–628.
Article PubMed Google Scholar - Ettinger U, Kumari V, Crawford TJ, Flak V, Sharma T, Davis RE et al (2005). Saccadic eye movements, schizotypy, and the role of neuroticism. Biol Psychol 68: 61–78.
Article PubMed Google Scholar - Ettinger U, Picchioni M, Hall MH, Schulze K, Toulopoulou T, Landau S et al (2006). Antisaccade performance in monozygotic twins discordant for schizophrenia: the Maudsley twin study. Am J Psychiatry 163: 543–545.
Article PubMed Google Scholar - Flint J, Munafo MR (2007). The endophenotype concept in psychiatric genetics. Psychol Med 37: 163–180.
Article PubMed Google Scholar - Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW (2003). DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav Genet 33: 67–72.
Article CAS PubMed Google Scholar - Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW (2007). Comment on ‘Wandering minds: the default network and stimulus-independent thought’. Science 317: 43.
Article CAS PubMed Google Scholar - Gilbert SJ, Simons JS, Frith CD, Burgess PW (2006). Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. J Exp Psychol Hum Percept Perform 32: 45–58.
Article PubMed Google Scholar - Gooding DC, Shea HB, Matts CW (2005). Saccadic performance in questionnaire-identified schizotypes over time. Psychiatry Res 133: 173–186.
Article PubMed Google Scholar - Gottesman II, Gould TD (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636–645.
Article PubMed Google Scholar - Grosbras MH, Laird AR, Paus T (2005). Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp 25: 140–154.
Article PubMed PubMed Central Google Scholar - Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264.
Article CAS PubMed PubMed Central Google Scholar - Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA (2007). Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci 27: 3477–3489.
Article CAS PubMed PubMed Central Google Scholar - Hariri AR, Weinberger DR (2003). Imaging genomics. Br Med Bull 65: 259–270.
Article CAS PubMed Google Scholar - Harrison BJ, Yucel M, Pujol J, Pantelis C (2007). Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res 91: 82–86.
Article PubMed Google Scholar - Heinz A, Smolka MN (2006). The effects of catechol _O_-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci 17: 359–367.
Article CAS PubMed Google Scholar - Hoffman JE, Subramaniam B (1995). The role of visual attention in saccadic eye movements. Percept Psychophys 57: 787–795.
Article CAS PubMed Google Scholar - Hong J, Shu-Leong H, Tao X, Lap-Ping Y (1998). Distribution of catechol-_O_-methyltransferase expression in human central nervous system. NeuroReport 9: 2861–2864.
Article CAS PubMed Google Scholar - Hutton SB, Ettinger U (2006). The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 43: 302–313.
Article PubMed Google Scholar - Jazbec S, McClure E, Hardin M, Pine DS, Ernst M (2005). Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry 58: 632–639.
Article PubMed Google Scholar - Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G et al (2002). Catechol-_O_-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin Card Sorting Test. Arch Gen Psychiatry 59: 662–663.
Article PubMed Google Scholar - Kennedy DP, Redcay E, Courchesne E (2006). Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci USA 103: 8275–8280.
Article CAS PubMed PubMed Central Google Scholar - Kumari V, ffytche DH, Williams SC, Gray JA (2004). Personality predicts brain responses to cognitive demands. J Neurosci 24: 10636–10641.
Article CAS PubMed PubMed Central Google Scholar - Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM (1996). Human catechol-_O_-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6: 243–250.
Article CAS PubMed Google Scholar - Lloyd KG, Davidson L, Hornykiewicz O (1975). The neurochemistry of Parkinson's disease: effect of L-dopa therapy. J Pharmacol Exp Ther 195: 453–464.
CAS PubMed Google Scholar - Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I et al (1995). Kinetics of human soluble and membrane-bound catechol _O_-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34: 4202–4210.
Article CAS PubMed Google Scholar - Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007). Wandering minds: the default network and stimulus-independent thought. Science 315: 393–395.
Article CAS PubMed PubMed Central Google Scholar - Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K et al (2004). Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res 131: 147–155.
Article PubMed Google Scholar - Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O et al (2001). Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298.
Article CAS PubMed Google Scholar - McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ et al (2002). Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry 51: 216–223.
Article PubMed Google Scholar - McGuffin P, Riley B, Plomin R (2001). Genomics and behavior. Toward behavioral genomics. Science 291: 1232–1249.
Article CAS PubMed Google Scholar - McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR (2003). A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408.
Article PubMed Google Scholar - Menon V, Leroux J, White CD, Reiss AL (2004). Frontostriatal deficits in fragile X syndrome: relation to FMR1 gene expression. Proc Natl Acad Sci USA 101: 3615–3620.
Article CAS PubMed PubMed Central Google Scholar - Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, Inerney-Leo A, Nussbaum R et al (2005). Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci 8: 594–596.
Article CAS PubMed Google Scholar - Meyer-Lindenberg A, Weinberger DR (2006). Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 7: 818–827.
Article CAS PubMed Google Scholar - Mitterschiffthaler MT, Ettinger U, Mehta MA, Mataix-Cols D, Williams SC (2006). Applications of functional magnetic resonance imaging in psychiatry. J Magn Reson Imaging 23: 851–861.
Article PubMed Google Scholar - Mort DJ, Perry RJ, Mannan SK, Hodgson TL, Anderson E, Quest R et al (2003). Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage 18: 231–246.
Article PubMed Google Scholar - Munafo MR, Bowes L, Clark TG, Flint J (2005). Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case–control studies. Mol Psychiatry 10: 765–770.
Article CAS PubMed Google Scholar - Munoz DP, Everling S (2004). Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218–228.
Article CAS PubMed Google Scholar - Müri RM, Heid O, Nirkko AC, Ozdoba C, Felblinger J, Schroth G et al (1998). Functional organisation of saccades and antisaccades in the frontal lobe in humans: a study with echo planar functional magnetic resonance imaging. J Neurol Neurosurg Psychiatry 65: 374–377.
Article PubMed PubMed Central Google Scholar - Nieman D, Becker H, van de FR, Plat N, Bour L, Koelman H et al (2007). Antisaccade task performance in patients at ultra high risk for developing psychosis. Schizophr Res 95: 54–60.
Article PubMed Google Scholar - Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A (2001). Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology 38: 752–760.
Article CAS PubMed Google Scholar - Nolan KA, Bilder RM, Lachman HM, Volavka J (2004). Catechol _O_-methyltransferase Val158Met polymorphism in schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry 161: 359–361.
Article PubMed Google Scholar - O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS (1995). Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci USA 92: 925–929.
Article PubMed PubMed Central Google Scholar - O’Driscoll GA, Lenzenweger MF, Holzman PS (1998). Antisaccades and smooth pursuit eye tracking and schizotypy. Arch Gen Psychiatry 55: 837–843.
Article PubMed Google Scholar - Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113.
Article CAS PubMed Google Scholar - Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Pierrot-Deseilligny C (2005). The prefrontal substrate of reflexive saccade inhibition in humans. Biol Psychiatry 57: 1159–1165.
Article PubMed Google Scholar - Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS (2005). Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci USA 102: 15700–15705.
Article CAS PubMed PubMed Central Google Scholar - Raemaekers M, Jansma JM, Cahn W, Van Der Geest JN, Der Linden JA, Kahn RS et al (2002). Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry 59: 313–320.
Article PubMed Google Scholar - Raemaekers M, Ramsey NF, Vink M, van den Heuvel MP, Kahn RS (2006). Brain activation during antisaccades in unaffected relatives of schizophrenic patients. Biol Psychiatry 59: 530–535.
Article PubMed Google Scholar - Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001). A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682.
Article CAS PubMed PubMed Central Google Scholar - Rammsayer T (2004). Extraversion and the dopamine hypothesis. In: Stelmack RM (ed). On the Psychobiology of Personality. Elsevier: Oxford. pp 409–428.
Chapter Google Scholar - Reuter B, Kathmann N (2004). Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychol (Amst) 115: 255–269.
Article Google Scholar - Rypma B, D’Esposito M (1999). The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci USA 96: 6558–6563.
Article CAS PubMed PubMed Central Google Scholar - Sauer J, ffytche DH, Ballard C, Brown RG, Howard R (2006). Differences between Alzheimer's disease and dementia with Lewy bodies: an fMRI study of task-related brain activity. Brain 129: 1780–1788.
Article PubMed Google Scholar - Savitz J, Solms M, Ramesar R (2006). The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav 5: 311–328.
Article CAS PubMed Google Scholar - Smyrnis N, Evdokimidis I, Stefanis NC, Avramopoulos D, Constantinidis TS, Stavropoulos A et al (2003). Antisaccade performance of 1273 men: effects of schizotypy, anxiety, and depression. J Abnorm Psychol 112: 403–414.
Article PubMed Google Scholar - Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR et al (1996). Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol 75: 454–468.
Article CAS PubMed Google Scholar - Talairach J, Tournoux P (1988). Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers: New York.
Google Scholar - The Stationary Office (2000). Standard Occupational Classification, Vol 2. London.
- Tinaz S, Schendan HE, Stern CE (2006). Fronto-striatal deficit in Parkinson's disease during semantic event sequencing. Neurobiol Aging; e-pub ahead of print 6 December 2006.
- Tu PC, Yang TH, Kuo WJ, Hsieh JC, Su TP (2006). Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res 40: 606–612.
Article CAS PubMed Google Scholar - Tunbridge EM, Harrison PJ, Weinberger DR (2006). Catechol-_O_-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry 60: 141–151.
Article CAS PubMed Google Scholar - Walker R, Husain M, Hodgson TL, Harrison J, Kennard C (1998). Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia 36: 1141–1159.
Article CAS PubMed Google Scholar - Wang WY, Barratt BJ, Clayton DG, Todd JA (2005). Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet 6: 109–118.
Article CAS PubMed Google Scholar - Weinshilboum RM, Otterness DM, Szumlanski CL (1999). Methylation pharmacogenetics: catechol _O_-methyltransferase, thiopurine methyltransferase, and histamine _N_-methyltransferase. Annu Rev Pharmacol Toxicol 39: 19–52.
Article CAS PubMed Google Scholar - Weissman DH, Roberts KC, Visscher KM, Woldorff MG (2006). The neural bases of momentary lapses in attention. Nat Neurosci 9: 971–978.
Article CAS PubMed Google Scholar - Winterer G, Goldman D (2003). Genetics of human prefrontal function. Brain Res Brain Res Rev 43: 134–163.
Article CAS PubMed Google Scholar - Winterer G, Musso F, Vucurevic G, Stoeter P, Konrad A, Seker B et al (2006). COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. NeuroImage 32: 1722–1732.
Article PubMed Google Scholar
Acknowledgements
This work was supported by funding from the Leverhulme Trust (ECF/2004/0370) and the ESRC/MRC (PTA-037-27-0002). Ulrich Ettinger is funded by an NIHR (National Institute for Health Research) Personal Award. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, or Department of Health. Veena Kumari is supported by a Wellcome Senior Research Fellowship. We thank Simon Surguladze and Sam Gilbert for helpful comments on a previous version of this manuscript.
Author information
Authors and Affiliations
- King's College London, Centre for Neuroimaging Sciences, Institute of Psychiatry, London, UK
Ulrich Ettinger, Olurotimi Zedomi & Steven C R Williams - Department of Psychology, King's College London, Institute of Psychiatry, London, UK
Veena Kumari - Division of Psychological Medicine, King's College London, Institute of Psychiatry, London, UK
David A Collier & Sonija Luzi - King's College London, Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, London, UK
David A Collier - Department of Neuroscience, King's College London, Institute of Psychiatry, London, UK
John Powell - Department of Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany
Tanja M Michel
Authors
- Ulrich Ettinger
You can also search for this author inPubMed Google Scholar - Veena Kumari
You can also search for this author inPubMed Google Scholar - David A Collier
You can also search for this author inPubMed Google Scholar - John Powell
You can also search for this author inPubMed Google Scholar - Sonija Luzi
You can also search for this author inPubMed Google Scholar - Tanja M Michel
You can also search for this author inPubMed Google Scholar - Olurotimi Zedomi
You can also search for this author inPubMed Google Scholar - Steven C R Williams
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toUlrich Ettinger.
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The authors state no conflict of interest.
Rights and permissions
About this article
Cite this article
Ettinger, U., Kumari, V., Collier, D. et al. Catechol-_O_-Methyltransferase (COMT) Val158Met Genotype is Associated with BOLD Response as a Function of Task Characteristic.Neuropsychopharmacol 33, 3046–3057 (2008). https://doi.org/10.1038/sj.npp.1301658
- Received: 25 July 2007
- Revised: 24 October 2007
- Accepted: 13 November 2007
- Published: 30 January 2008
- Issue Date: December 2008
- DOI: https://doi.org/10.1038/sj.npp.1301658