SNARE the rod, coil the complex (original) (raw)
- News & Views
- Published: 24 September 1998
Membrane fusion
Nature volume 395, pages 328–329 (1998)Cite this article
- 482 Accesses
- 43 Citations
- Metrics details
Despite the continuous flux of proteins and lipids within a eukaryotic cell, its membrane-bound compartments maintain distinct identities. Their composition is regulated by the transport of cellular cargo from one organelle to another within membrane-bound vesicles — a region of the donor membrane is pinched off, then it fuses with the target membrane and delivers the cargo. This process is governed by a highly conserved machinery that includes proteins on both the vesicle and target membranes, known respectively as v- and t-SNAREs (soluble _N_-ethylmaleimide-sensitive factor attachment protein receptors). Sutton et al.1 (on page 347 of this issue) and Poirier et al.2 (in Nature Structural Biology) now take a step towards understanding the function of SNARE complexes, by determining the structure of the regions that are important in forming the core fusion complexes.
The cytoplasmic portions of SNARE proteins contain several α-helical regions, with heptad (seven-amino-acid) repeats in their sequences. The first and fourth residues of each repeat are hydrophobic amino acids, so one face of the α-helix is hydrophobic. When the four helices of the core fusion complex interact, these faces pack against one another to produce the hydrophobic core of a coiled coil. The core fusion complex contains three proteins. A neuronal v-SNARE called synaptobrevin (also known as VAMP) and a neuronal t-SNARE known as syntaxin each contribute a single parallel helix to the structure3,4. Another neuronal t-SNARE, SNAP-25 (synaptosome-associated protein of relative molecular mass 25,000), contributes two helices, but the orientation of these helices was not known.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
v-SNARE function in chromaffin cells
- Madhurima Dhara
- , Ralf Mohrmann
- & Dieter Bruns
Pflügers Archiv - European Journal of Physiology Open Access 08 September 2017
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Figure 1: Models of SNARE function.
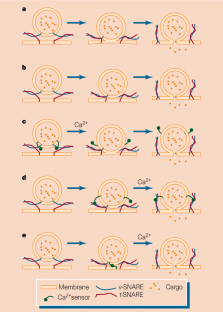
References
- Sutton, R. B., Fasshauer, D., Jahn, R. & Brunger, A. T. Nature 395, 347–353 (1998).
Article ADS CAS Google Scholar - Poirier, M. A. et al. Nature Struct. Biol. 5, 765–769 (1998).
Google Scholar - Hanson, P. I., Roth, R., Morisaki, H., Jahn, R. & Heuser, J. E. Cell 90, 523–535 (1997).
Google Scholar - Lin, R. C. & Scheller, R. H. Neuron 19, 1087–1094 (1997).
Google Scholar - Weimbs, T., Mostov, K. E., Low, S. H. & Hofmann, K. A. Trends Cell Biol. 8, 260–262 (1998).
Google Scholar - O'Shea, E. K., Rutkowski, R. & Kim, P. S. Cell 68, 699–708 (1992).
Google Scholar
Author information
Authors and Affiliations
- the Department of Structural Biology, Stanford, 94305, California, USA
William I. Weis - Department of Molecular and Cellular Physiology,
Richard H. Scheller - Howard Hughes Medical Institute, Stanford University School of Medicine, Stanford, 94305, California, USA
Richard H. Scheller
Authors
- William I. Weis
- Richard H. Scheller
Rights and permissions
About this article
Cite this article
Weis, W., Scheller, R. SNARE the rod, coil the complex.Nature 395, 328–329 (1998). https://doi.org/10.1038/26354
- Issue Date: 24 September 1998
- DOI: https://doi.org/10.1038/26354